

BioDesign™, by Vector Laboratories, provides extensive crosslinker offerings for use in applications where precision is required. One of these offerings is our proprietary dPEG® linkers, which are synthesized from high purity building blocks in a series of stepwise reactions to provide specific molecular weights, organic moieties, functional groups, and architectures. These products are part of our click chemistry crosslinking reagents and are well suited for a variety of uses.
The term “dPEG®” is Vector Laboratories’ trademarked acronym for “discrete polyethylene glycol” or “discrete PEG“. The “discrete” portion of the dPEG trademark indicates single molecular weight PEG technology and can help simplify product analysis. Comparatively, traditional PEGs are not single compounds.
Like traditional PEGs, our products contain an amphiphilic backbone of repeating ethylene oxide units. The term “amphiphilic” means that the compound is soluble in both water (or aqueous buffer) and organic solvents. All PEG products that do not contain hydrophobic substituents are soluble in water and in a variety of organic solvents. The addition of hydrophobic groups to PEG reduces the water solubility of some PEG products.
Vector Laboratories developed and continues to manufacture these monodisperse PEG products using our proprietary synthetic and purification processes.
Numerous compounds and macromolecular constructs can be synthesized via click chemistry, thus solving many synthetic chemistry problems. These products include organic and organometallic nanoparticles, dendrimers, small molecule drugs, peptide drugs, and antibody-drug conjugates (ADCs).
Click chemistry qualifies as environmentally friendly “green chemistry” due to its use of benign or easily removable solvents [2]. Additionally, typical click chemistry reactions run faster in water than in organic solvents [1]. Research and experience demonstrate that click chemistry can be a highly useful synthetic process for reactions that require aqueous environments, such as cell-based assays [3]. Unfortunately, some types of click chemistry – for example, copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) – require cytotoxic metal salts. However, strain-promoted azide-alkyne cycloaddition (SPAAC), avoids these harmful salts.
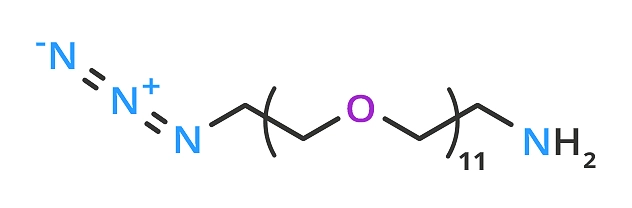
Numerous click chemistry crosslinking products are available in the BioDesign portfolio, including azido-dPEG®11-amine (QBD-10524) (Figure 1). This dPEG reagent has an azide group and a primary amine residing on opposite ends of a single molecular weight PEG linker (molecular weight of 570.67 Daltons; linker length of 36 atoms, ≈42.7 Å).
This product crosslinks carboxylic acids with alkyne-containing compounds. The amine end of the molecule forms amide bonds with carboxyl groups directly using a carbodiimide such as EDC. More commonly, the amide bond forms by reacting the amine with an active ester such as an N-hydroxysuccinimidyl (NHS) or 2,3,5,6-tetrafluorophenyl (TFP) ester. On the opposite end of the linker, the azide group reacts with alkyne-containing molecules via click chemistry reactions such as CuAAC or SPAAC. Please see Scheme 1 below.
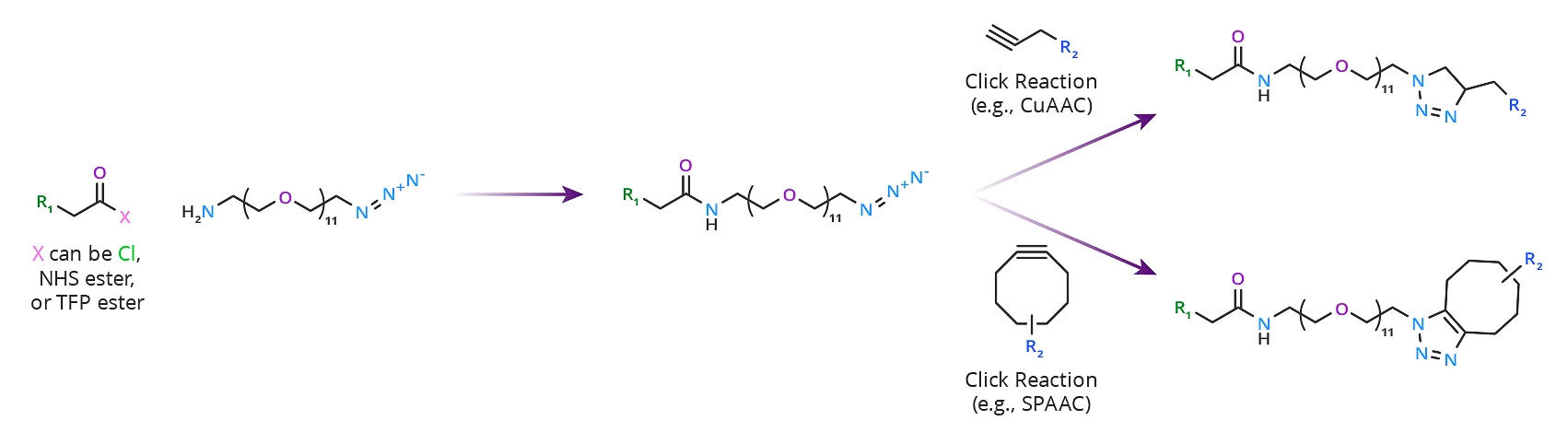
Scheme 1: Click chemistry crosslinking reactions using azido-dPEG®11-amine. In this scheme, both copper-catalyzed and copper-free click chemistry reactions are shown.
Surface Functionalized with Carboxylic Acid Groups
Surface now PEGylated and azidoated for click chemistry reactions
Figure 2: Surface coating with azido-dPEG®11-amine.
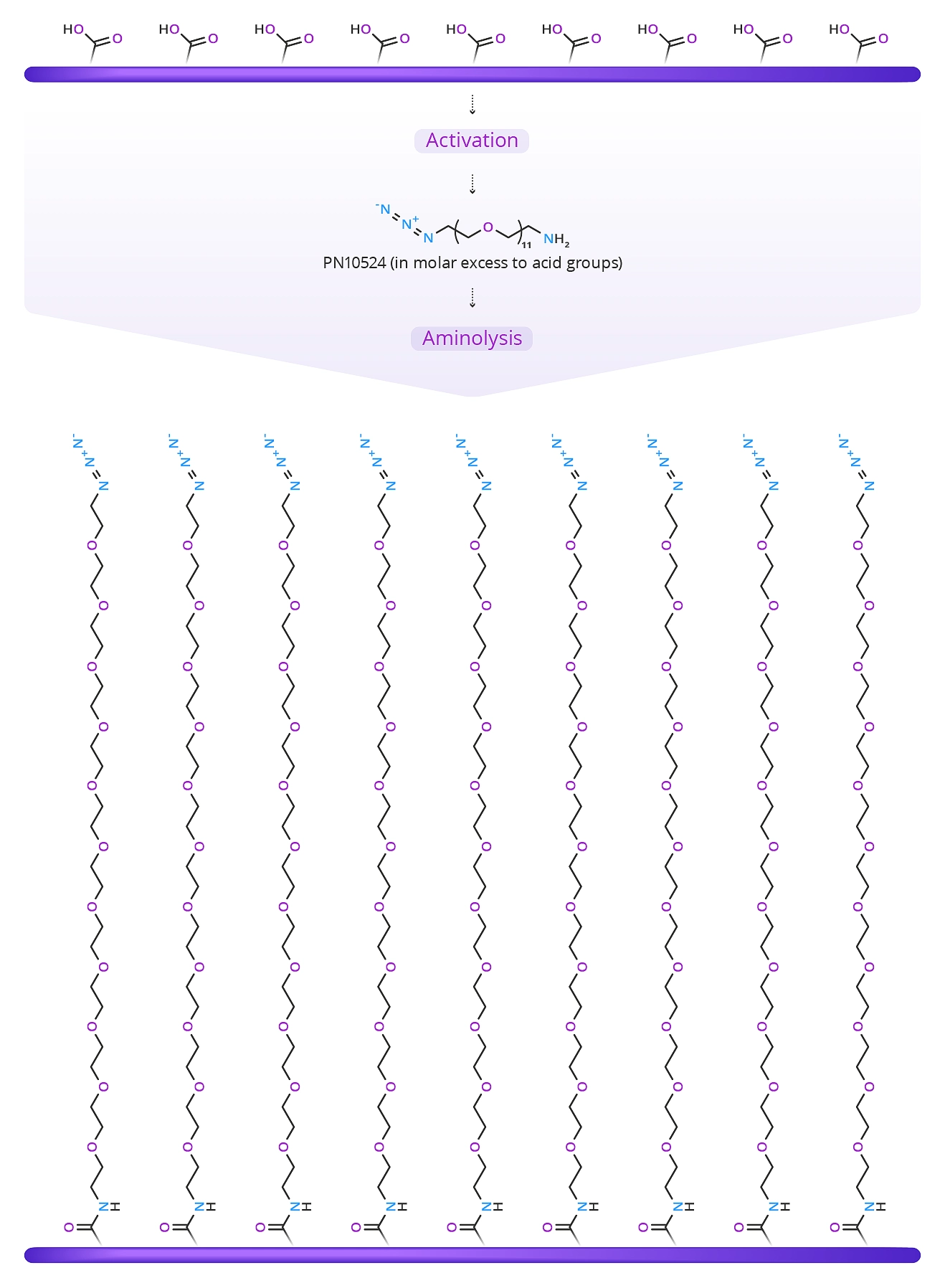
A dense coating of dPEG molecules reduces or eliminates non-specific binding. This coating, though, leaves many closely spaced azide groups sticking up from the surface. These azide groups can react with some target molecules (a small molecule, peptide, or protein into which an alkyne group has been installed). However, steric hindrance (crowding) prevents most of the azide groups from reacting with the target alkyne. The unreacted azides are effectively wasted.
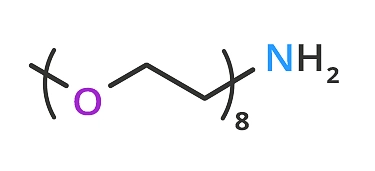
Figure 3: Product QBD-10278 m-dPEG®8-amine.
A better way to coat the surface is to mix the crosslinker with a methoxy-terminated dPEG amine. In this scenario, combining m-dPEG®8-amine (QBD-10278) (29.7 Å )(Figure 3) in a ratio of >3:1 with azido-dPEG®11-amine (44.2 Å ) crosslinker will achieve the results shown in Figure 4.
Surface Functionalized with Carboxylic Acid Groups
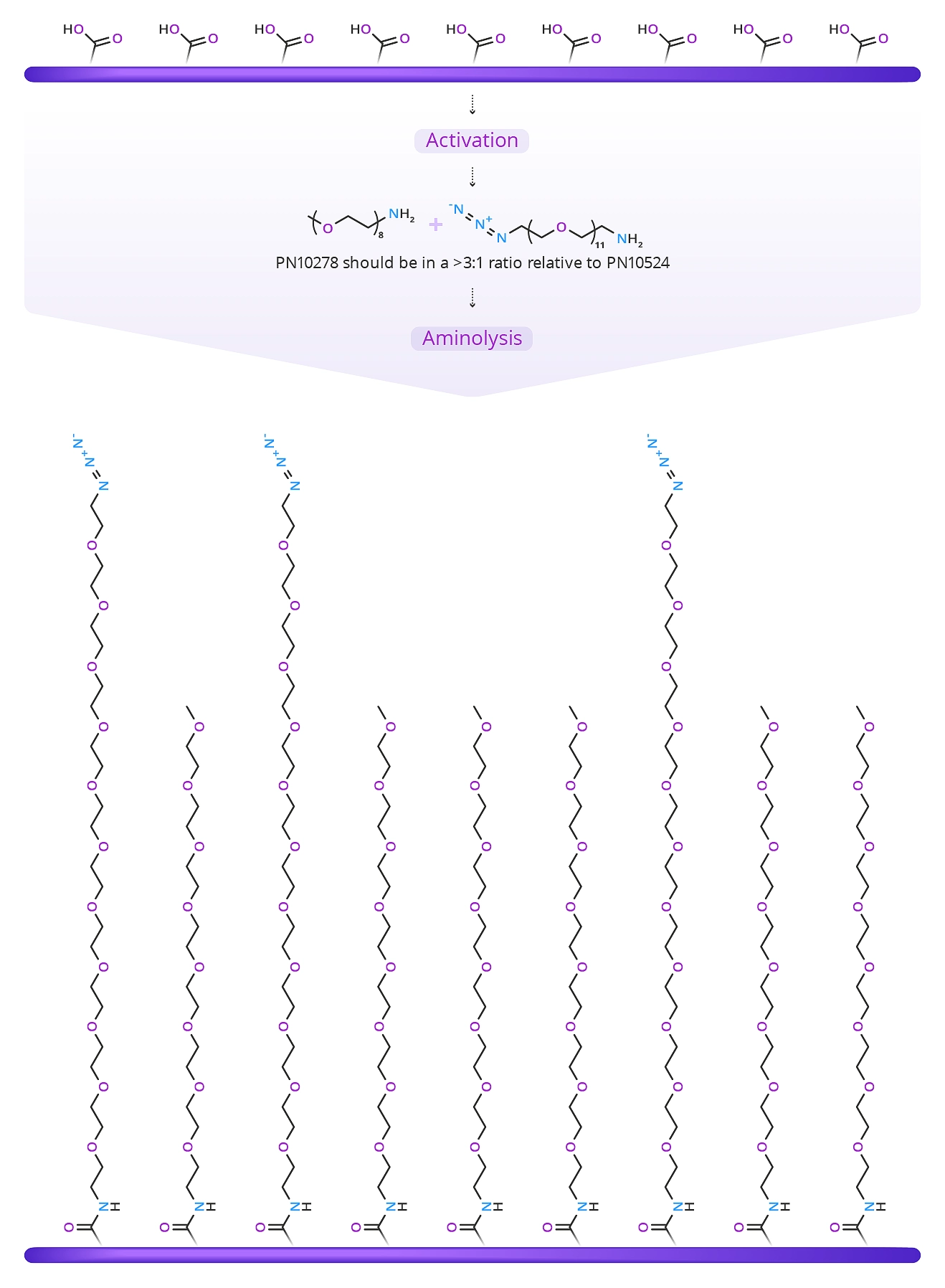
Surface now PEGylated, partly azidoated for click chemistryreactions, and fully blocked with m-DPEG®8 to prevent non-specific interactions with the surface.
This type of coating reduces non-specific binding. Fewer azide groups on the surface result in less crowding of the subsequent alkyne reactants. In turn, reduced steric crowding facilitates the introduction of large molecules, such as peptides and proteins, onto the surface. Thus, the decreased steric hindrance may prove particularly advantageous when carrying out SPAAC click chemistry with cyclooctyne groups that are often bulky.
The result was a well-defined macromolecular structure with precise spatial control that appeared from modeling studies to be able to inhibit the formation of anthrax toxin (Figure 6).
In testing the heptavalent anthrax toxin inhibitor, six out of seven rats treated with the inhibitor and exposed to anthrax did not develop anthrax. Conversely, rats exposed to anthrax and either not treated with inhibitor or treated with a placebo inhibitor developed anthrax symptoms and died.
CuAAC is a simple reaction that gives high yields with minimal byproducts. This research shows the power of using click chemistry with a dPEG reagent to exert spatial control over macromolecular product design. The precise spatial control needed to position the inhibiting peptide from the β-cyclodextrin core would not have been possible with a dispersed PEG. Only with a single molecular weight PEG (dPEG) could that kind of control been obtained. Having a single molecular weight PEG product allows greater control over product design and purity. As demonstrated in the example, compared to traditional, dispersed PEGs, a dPEG compound simplifies product analysis.
Atomic Force Microscopy (AFM) is a type of scanning probe microscopy with a resolution to fractions of a nanometer. One application of AFM measures the force between a probe and a sample. This use of AFM is known as force spectroscopy, which is when “single (bio-) molecular bonds are actively broken to assess their range and strength” [9].
In single-molecule AFM force spectroscopy (SMFS), compounds of interest are attached by linkers to the cantilevers used to measure the force. Each cantilever attaches a single molecule [9], hence the name.
PEG is the most commonly used linker for attaching biomolecules to cantilevers [11] [12] [13]. However, PEG polymers are dispersed and have varied chain lengths [12] [14], making traditional PEG linkers less than ideal for SMFS.
A 2012 Master’s Thesis by Jamie Maciaszek in the lab of Yuri L. Lyubchenko reported that linkers containing a single molecular weight and chain length were superior to traditional, dispersed PEG linkers [14]. Moreover, using azido-dPEG®11-amine SMFS research in the lab of George Lykotrafitis detected and quantitatively mapped individual calcium-activated small conductance (SK) potassium channels in living neurons. The researchers joined the amine end of the crosslinker to APTES-activated silicon nitride cantilevers. Click chemistry crosslinking then linked the molecules of interest with the resulting azide-coated surface. The dPEG linker was ideal for the research due to the absence of chain length heterogeneity [15].
BioDesign click chemistry crosslinkers are designed for versatility and can be utilized in an assortment of applications. dPEG products, including azido-dPEG®11-amine crosslinker, are available in different sizes to better suit specific requirements. Learn more about BioDesign and additional crosslinker options here.



Stay in the Loop. Join Our Online Community
Products
Ordering
About Us
Application
Resources

©Vector Laboratories, Inc. 2025 All Rights Reserved.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Privacy Statement
Figure 1: Azido-dPEG®11-amine: a BioDesign click chemistry crosslinking reagent.