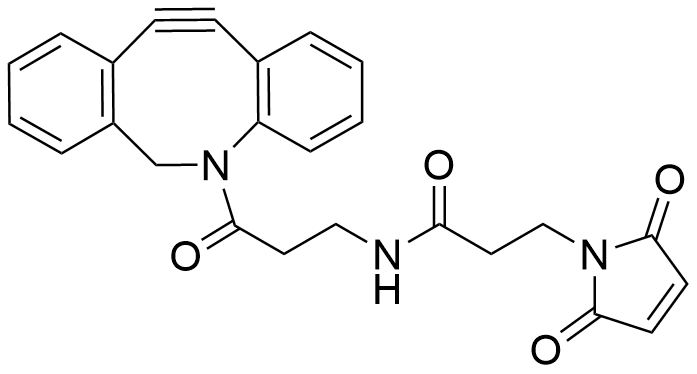
Bioconjugation
Conjugate with confidence
Leverage bioconjugation to expand your molecular toolbox and create the perfect match for your research needs.
Vector Labs offers a wide variety of linkers, dyes and comprehensive kit formats to generate bioconjugates. Whether your research requires traditional biotin or NHS ester linkers or more recently developed copper-free catalyzed click chemistry, products in our extensive portfolio have been used in many applications across the biological sciences.
Our products are designed for high-performance conjugation of all classes of biomolecules including antibodies, proteins, peptides, oligonucleotides, drugs and surfaces.
Explore our bioconjugation products:

Fully Integrated Kits
From conjugation to purification, our fully integrated kits ensure the generation of high-quality conjugates, free of unincorporated label.

Reliable
With mild reaction conditions and high affinity conjugate chemistry, you’ll generate consistent results with an unparalleled level of reproducibility across experiments.
Flexible
With versatile technology to conjugate and capture all biomolecules, our product portfolio and expertise are available to ensure successful scaleup of your conjugation needs from micrograms to grams to kilos.
High Yield
Leverage efficient labeling, without a need for heavy metal catalysts, to prepare biomolecule conjugates at high yield with uncompromised purity.

Quantifiable
Monitor and record conjugation progress in real-time with a UV-traceable chromophore, enabling direct quantification to confirm experimental success.
Webinar: An Introduction to Bioconjugation
Bioconjugation Resource Guide: Methodology to Linking Technology
This guide provides an overview of bioconjugation, applications and uses, as well as tools for successful bioconjugation.
Quantitative and Reproducible Bioconjugation with SoluLINK Technology
Explore the bioconjugation workflow and considerations for selecting a conjugation strategy.


Webinar: An Introduction to Bioconjugation
Bioconjugation Resource Guide: Methodology to Linking Technology
This guide provides an overview of bioconjugation, applications and uses, as well as tools for successful bioconjugation.

Quantitative and Reproducible Bioconjugation with SoluLINK Technology
Explore the bioconjugation workflow and considerations for selecting a conjugation strategy.