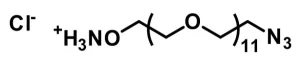
Azido-dPEG®11-amine, product number QBD-10524, is a carbonyl-reactive, biocompatible crosslinking reagent that can participate in multiple types of reactions. The product consists of azide and amine groups on opposite ends of a medium-length (36 atoms), single molecular weight, discrete polyethylene glycol (dPEG®) chain. Alkynes react with the azido group in metal-catalyzed or strain-promoted click chemistry to yield triazole derivatives. Moreover, the azide group functions as a masked amine that reduces to a primary amine via a suitable reducing agent. Furthermore, Azido-dPEG®11-amine can participate in a Staudinger ligation with appropriate phosphine derivatives to create a water-soluble product.
The amphiphilic dPEG® product adds hydrodynamic volume and imparts water solubility to any compound to which it is conjugated. Also, because the terminal azide group functions in multiple different types of reactions, Azido-dPEG®11-amine can act as either a heterobifunctional, click chemistry crosslinker or a monoprotected homobifunctional crosslinking reagent. As a heterobifunctional click chemistry reagent, the azide group reacts with alkynes to form triazoles. The amino terminus of the molecule then can react with carboxylic acids, aldehydes, or ketones to crosslink the conjugate to another molecule. As a monoprotected homobifunctional crosslinker, the amine end reacts first with carboxylic acids (or their active esters), aldehydes, or ketones, leaving the azide end of the molecule free. The reduction of the azido group with a suitable reducing agent, such as triphenylphosphine, yields a free amine that can be conjugated similarly to the conjugation of the first amine group. Reacting the amine groups with aldehydes or ketones results in labile Schiff bases that should be reduced to secondary amines for bond stability.
| Unit Size | 100 mg, 1000 mg |
|---|---|
| Molecular Weight | 570.67; single compound |
| Chemical formula | C₂₄H₅₀N₄O₁₁ |
| CAS | 749244-38-0 |
| Purity | > 98% |
| Spacers | dPEG® Spacer is 36 atoms and 44.2 Å |
| Shipping | Ambient |
| Typical solubility properties (for additional information contact Customer Support) | Methylene chloride, Acetonitrile, DMAC, or DMSO. |
| Storage and handling | -20°C; Always let come to room temperature before opening; be careful to limit exposure to moisture and restore under an inert atmosphere; stock solutions can be prepared with dry solvent and kept for several days (freeze when not in use). dPEG® pegylation compounds are generally hygroscopic and should be treated as such. This will be less noticeable with liquids, but the solids will become tacky and difficult to manipulate, if care is not taken to minimize air exposure. |
Greg T. Hermanson, Bioconjugate Techniques, 3rd Edition, Elsevier, Waltham, MA 02451, 2013, ISBN 978-0-12-382239-0; See Chapter 18, Discrete PEG Reagents, pp. 787-821, for a full overview of the dPEG® products.
Structure-Based Design of a Heptavalent Anthrax Toxin Inhibitor. Amit Joshi, Sandesh Kate, Vincent Poon, Dhananjoy Mondal, Mohan B. Boggara, Arundhati Saraph, Jacob T. Martin, Ryan McAlpine, Ryan Day, Angel E. Garcia, Jeremy Mogridge, and Ravi S. Kane. Biomacromolecules, 2011, 12 (3), pp 791–796 February 8, 2011. DOI: 10.1021/bm101396u.
Detection of SK2 Channels on Hippocampal Neurons. Jamie L. Maciaszek University of Connecticut (2012) DigitalCommons@Uconn Master’s Theses Paper 237 April 20, 2012. http://digitalcommons.uconn.edu/gs_theses/237.
Applicable patents and legal notices are available at legal notices.
Stay in the Loop. Join Our Online Community
Products
Ordering
About Us
Application
Resources

©Vector Laboratories, Inc. 2025 All Rights Reserved.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Privacy Statement
How do I Request a Quote?
To request a quote for products: