
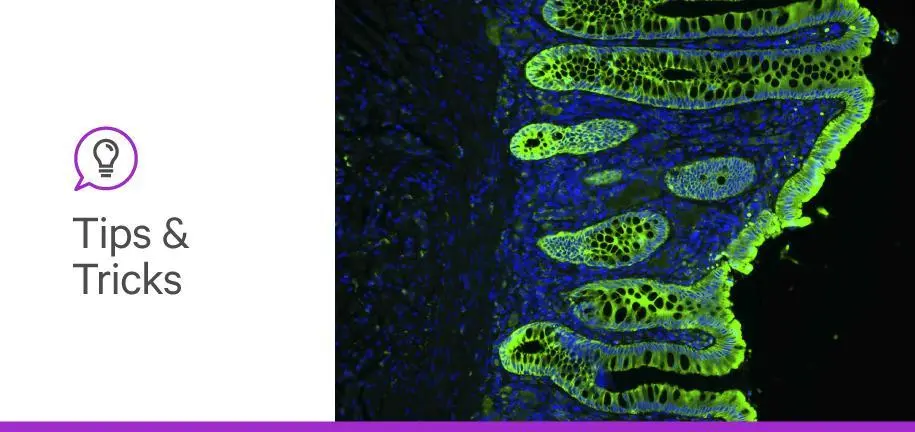
Antifade mounting media, or solutions in which biological samples are embedded for imaging, play a sometimes unappreciated role in science. Though often thought of merely as agents that preserve biological specimens for later imaging, antifade mounting media can do so much more. They can improve the detailedness and fidelity of your microscopy images, enable certain microscopy applications, and even prevent photobleaching (fading of fluorescence).
As far as antifade mounting media go, one-size-fits-all solutions are few and far between. Where one may perform better, another may not. Here, we break down 5 key factors you should consider when choosing the best antifade mounting medium for your experiment.
From epifluorescence microscopy to whole-mount imaging, every fluorescence microscopy experiment begins with a sample—the biological entity you plan to visualize. The first thing you should consider when deciding on an antifade mounting medium is how the light from your microscope interacts with your sample. One way to do this is by comparing the refractive index of your sample with those of different slide mounting media and the other components of your prepared sample (e.g., glass).
As light passes through one medium into another, it refracts or bends. You can see this when you put the end of a stick into water. It may appear that the stick is bending when, in reality, it’s only the image of the stick that’s bending.
You may have also noticed your tissue sections becoming more and more transparent as you move them from air to aqueous buffer to mounting medium. These apparent changes arise from differences in the refractive indices of air (1.00), water (1.33), and mounting media (1.47) (1).
When imaging biological structures, the more detail you can see, the more confident you can be in your scientific conclusions. Two parameters are key to obtaining a highly detailed and unambiguous fluorescent image: clarity and contrast. Clarity refers to the detailedness of an image whereas contrast refers to the difference in light intensity between staining and unstained structures (background).
To maximize these parameters, you should try to match the refractive indices of all the components of your prepared samples: the coverslip, the mounting medium, and the biological sample itself. Glass has a refractive index of ~1.50, and the refractive index of fixed tissue falls somewhere between 1.36 and 1.53 (1,2). Choosing a mounting medium close to 1.50 will render the unstained parts of your prepared sample largely transparent, allowing you to better visualize the fluorescently stained areas.
Many fluorescence mounting media incorporate the organic solvent glycerol because it is cheap, safe, easy to use, and has a refractive index that is ideal for imaging biological specimens (~1.47).
Another thing to consider is whether your experiment calls for a setting antifade medium, such as VECTASHIELD Vibrance® Antifade Mounting Media, or non-setting (liquid) antifade mounting medium, such as VECTASHIELD® PLUS Antifade Mounting Media. A mounting medium that sets, while useful for long-term storage and repeated imaging, must be cured over the course of hours to days before it is ready for imaging. By contrast, a non-setting medium obviates the need for curing, making it useful for immediate imaging.
You can read more about the differences between setting and non-setting media in our post Setting media vs non-setting mounting media: Which is right for you?
Mounting media can impact the performance of fluorophores, or molecules that emit light (fluorescence) upon light excitation. Factors that influence fluorescence emission include the pH, ionic strength, and viscosity of the mounting media (1). Many mounting media are made alkaline (basic) to improve fluorescence emission (3).
The antifade reagents included in mounting media can also adversely impact select fluorophores. For example, the commonly used antifade reagent p-phenylenediamine (PPD) causes autofluorescence, making it less suitable for detection of light with excitation wavelengths <500 nm (i.e., detection of blue/green fluorophores) (4).
Thankfully, commercial mounting media vendors do the hard work for you by listing fluorophore compatibility in product documentation, allowing you to easily check whether a mounting media will work for your application. You may also find it worthwhile to consult the literature for more information.
Perhaps one of the most important factors to consider when choosing an antifade mounting medium is its antifade properties!
Exposure to intense excitation light can cause fluorophores to dim over time, or photobleach. The interaction of excited fluorophores with oxygen creates free radicals that can damage fluorophores such that they no longer fluoresce. Antifade mounting media prevent this with free radical scavenging compounds that dramatically slow down photobleaching.
Examples of antifading reagents include 1,4-diazobicyclo-[2,2,2]-octane (DABCO), p-phenylenediamine (PPD), n-propyl gallate (NPG), and sodium azide (5). PPD is generally considered one of the more effective antifading reagents but it is prone to autofluorescence and quenching by common detergents used to permeabilize cells and tissue (e.g., Triton-X). These issues can be circumvented by using red fluorophores and ensuring that any detergents applied to samples are thoroughly washed away before applying mounting media.
Finally, some fluorescent microscopy applications have unique requirements that impact the choice of antifade mounting medium.
Let’s say you want to acquire a high-resolution 3D image (xyz-stack) of your sample. You could obtain one using a confocal microscope equipped with an immersion objective that requires an immersion medium between the slide and lens. In this example, you would be wise to choose an immersion medium with a refractive index as close as possible to your mounting medium (or vice versa).
A refractive index mismatch can result in an elongation of the fluorescent signal in the vertical (z) plane, a phenomenon known as spherical aberration. This phenomenon occurs when a refractive index mismatch causes light to deviate from the focal point in your sample. Oil is commonly used as an immersion medium because it has a refractive index of ~1.5.
Applications that require high irradiation intensities such as super-resolution microscopy require mounting media that contains antifade reagents. Without these reagents, high-intensity light can cause the emission spectra of fluorophores to shift towards shorter (bluer) wavelengths (6). This photoblueing phenomenon occurs via the same molecular pathways as photobleaching.
As you can see, the choice of antifade mounting media is an important one. Failing to consider the factors outlined here could result in a blurry or faded microscope image at best and having to completely repeat your experiment at worst. Now that you know the factors that influence the performance of antifade mounting media, you can determine which medium is right for your experiment.
If you’d like to learn more about antifade mounting media and immunofluorescence, check out our Immunofluorescence Guide, and be sure to stay tuned for more tips and tricks here on the blog.




Stay in the Loop. Join Our Online Community
Products
Ordering
About Us
Application
Resources

©Vector Laboratories, Inc. 2025 All Rights Reserved.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Privacy Statement