
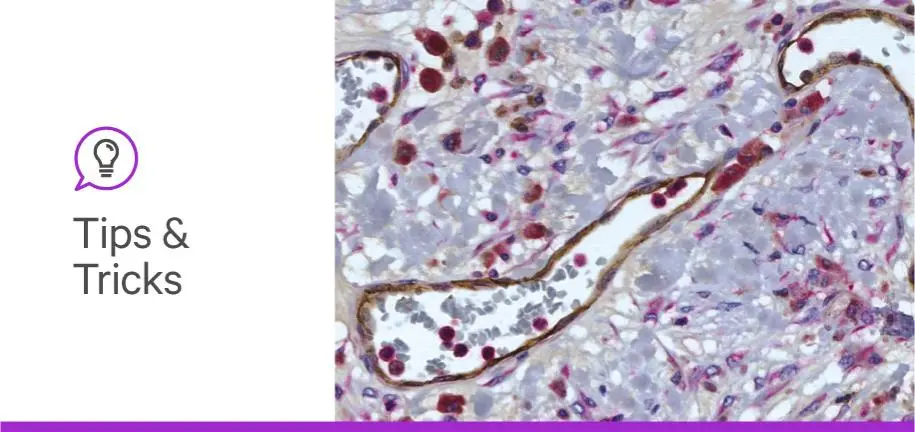
Investigators are leveraging multiplex immunohistochemistry (IHC) to tackle new research frontiers. From developing personalized diagnostics for melanoma patients to teasing out the immune microenvironment in colon cancer, the simultaneous visualization of multiple targets on the same specimen can help solve an incredible array of scientific questions (1,2). Curious about where this versatile technique could take your science? You’re in the right place! Keep reading for some Vector Laboratories-approved insider knowledge to make your multiple-antigen staining sparkle. For even more hot tips and tricks, check out Dr Craig Pow’s full-length webinar, “Multiplex Immunohistochemstry — Expand Your Insights, Improve Your Results”, and our other posts.
First things first: once you have carefully picked out your antibodies and enzyme detection systems (for advice on how to choose wisely, we have a blog post on getting started with multiplexing IHC), in what order should you apply them to your specimen? Throwing them on willy-nilly can undo all your meticulous planning, so allow us to share a few wise words from Vector Laboratories’ multiplex maven Craig, who earned his PhD in Physiology from the University of Queensland. He suggests always labeling the less prevalent antigen first and pairing it with the most sensitive detection system. Then, follow up with your second label for the more strongly expressed target. Of course, if you have more than two labels, you would stain them in order from the least to most abundant antigen. Does combining multiple substrates and figuring out how best to apply them leave you scratching your head? We put together a susbstrate selection page that makes it a breeze.
Next up, controls. You will need both positive and negative single-label controls for each target antigen and corresponding detection system (“The Curious Case of the Staining that Went Wrong” is a great refresher on running stellar single-stain controls). Run them in parallel—still as single labels—on separate test specimens. You will really understand where you should be seeing positive and negative results on your multiplex specimen. This will give you confidence that you’re seeing a true multiple staining result in your multiplex stain. Craig recommends running single-label stains in parallel each time that you perform multiplex immunohistochemistry. Otherwise, you may be unsure of whether you’re looking at a novel observation or a pesky false result!
You have yielded negative controls to find out how stained tissue that doesn’t express your antigen of interest looks. Now, you will use them to sniff out any potential false-positive staining caused by cross-reactivity between your multiple labels. Start by running your first stain all the way through, including first primary antibody, first secondary, and first enzyme substrate. If it looks great, continue with your second stain, but substitute a non-immune IgG for your second primary antibody (non-immune IgG + second secondary antibody + second enzyme substrate). If you see a signal from your second label with the non-immune IgG, it could mean that either your second secondary antibody is binding to excess first primary antibody or that the second enzyme substrate is reacting with residual enzyme—this can happen if you use the same enzyme-based detection system for multiple stains, like two peroxidase-based systems. To figure out what’s going on, run your first stain all the way through again and then apply the second enzyme substrate alone. Still getting a signal? Consider adding a step to quench residual enzyme activity between your first and second stains. For workflows that call for a tertiary label, simply repeat the process with your third stain.
Using a two-step methodology or an avidin-biotin complex kit? The same principles apply. Just as with the previous example, start by applying your first label, including first primary antibody, first secondary antibody, the biotinylated secondary avidin/streptavidin-based enzyme system, and first substrate. Then, run your second stain to completion, substituting a non-immune IgG for your second primary antibody as before. If you see a signal with the non-immune IgG, eliminate each step of your protocol systematically: the second secondary antibody, then the second biotinylated secondary avidin/streptavidin-based enzyme system, and finally the second substrate. Once you have worked out where the trouble lies and resolved the issue, you will feel confident about your staining.
You have done all your controls, great! But what if your perfect staining is still being marred by inherent enzyme activity? Craig explains that this is particularly likely to happen with peroxidase-based detection systems and that some researchers circumvent the issue by using only alkaline phosphatase-based detection systems. However, there are many reasons to choose one detection system over another, and we would hate to see you limited by inherent enzyme activity. Instead, quench the endogenous enzyme activity with a blocking solution. Consider simplifying your workflow with a universal block like BLOXALL® Endogenous Blocking Solution, which knocks out inherent peroxidase and alkaline phosphatase enzyme activity in one fell swoop and is compatible with all primary antibodies.
Your thoughts may turn to counterstains as you approach the end of your staining protocol, but Craig cautions that unless used carefully, a counterstain can detract from your specific antigen staining by masking your staining or interfering with the interpretation of your results. This is especially true if your specimen already has a lot of signal. He suggests that multiplexers avoid falling into the trap of lab dogma when following an established protocol and consider whether a counterstain is truly needed. “You can really ruin a lot of hard work [with a haphazard counterstain],” he explains. If you decide to go ahead, be very careful with the application. A light and balanced counterstain may help your specimen shine, while a heavy counterstain could muddy your results. Think carefully about the localization of your target antigen(s), too. For example, if one of your antigens is localized to the nucleus and you use a nuclear counterstain, be sure that you have a good demarcation between the counterstain and the label itself. Finally, always optimize the counterstain on separate tissue sections by troubleshooting experimental parameters such as application time and pH before applying it to your precious specimen. Ready to take the leap? Head on back to that gorgeous substrate selection page you looked at earlier to get the scoop on matching substrates to compatible counterstains!
Now that you’re a multiplex immunohistochemistry pro, you may be thinking about how to reliably stain co-localized antigens. Keep a few things in mind to achieve the experimental results of your dreams. If you have 100% overlap, meaning that every cell positive for one antigen is also positive for the other, interpretation can be very challenging. Craig recommends steering clear of 100% overlap to avoid this headache. Next, remember that peroxidase and alkaline phosphatase have different physical characteristics and precipitate out in different ways. Alkaline phosphatase-based substrates yield a more diffuse and transparent stain, while peroxidase-based substrates result in a dense, punctate precipitate. Reflect on which type of system will work best for your specific co-localization experiment. Lastly, don’t forget about the order of staining—as we mentioned earlier, always target your most weakly-expressed antigen with your most sensitive detection system first.
Follow these tips and you will be writing up your results in no time. Circle back to the SpeakEasy Science Blog soon for more expert advice to sharpen your skills at the bench, inspiring scientist stories that will brighten your day, and publication highlights to help spark your next big idea.




Stay in the Loop. Join Our Online Community
Products
Ordering
About Us
Application
Resources

©Vector Laboratories, Inc. 2025 All Rights Reserved.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Privacy Statement