
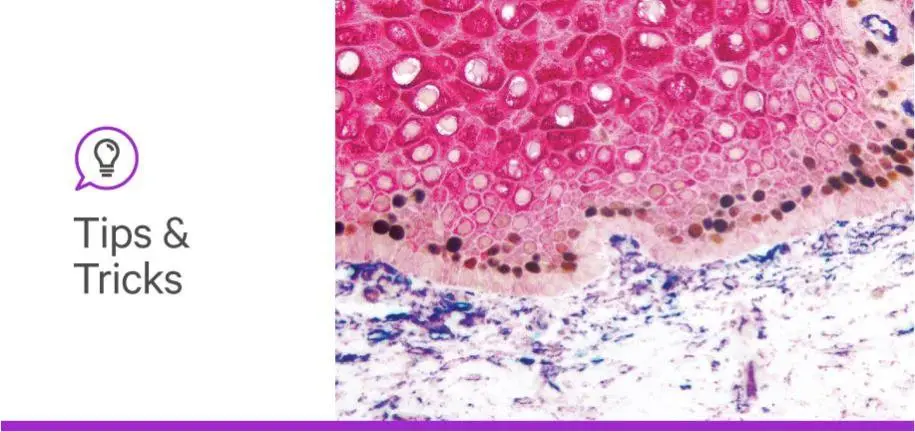
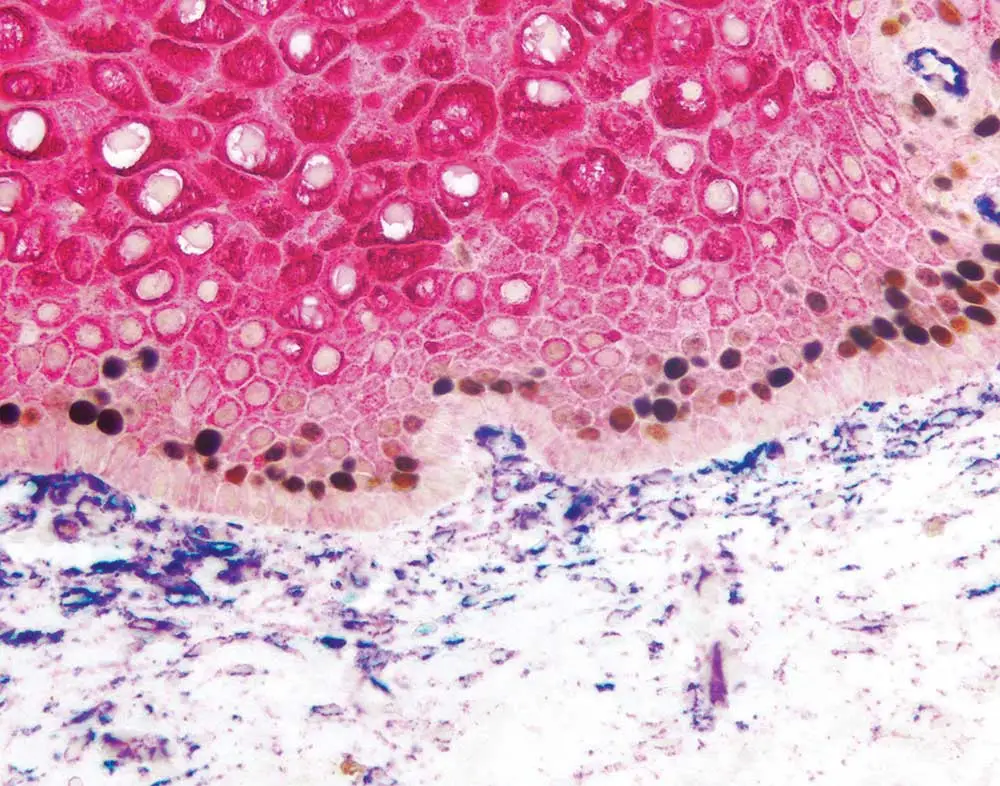
Multiplex immunohistochemistry, in which multiple antigens are labeled on the same tissue using enzyme-based substrates, can help researchers delve into an array of scientific topics including spatial relationships, intracellular signaling, and the tumor microenvironment. However, it can be a little daunting to incorporate additional antibodies into your IHC protocol. Don’t give up — we’re here to help! Take a look at this recent webinar from Dr. Craig Pow of Vector Laboratories to learn his double- and triple-staining secrets, or keep reading for some tips and tricks that are sure to take your immunostaining to the next level.
Successful, reliable, reproducible localization can certainly be obtained with three colors. You could potentially add one more, but four colors is likely to be the maximum for unequivocal chromogenic staining. Using more than four colors would probably lead to problems with background due to cross-reactivity.
A key factor is the length of time that the stained specimens need to be archived. Often, ongoing studies stretch over years, and people come and go from a lab during the course of a project. Chromogenic multiplex immunohistochemistry allows you to keep a specimen for a long period of time so that different segments can be revisited or higher magnification images can be taken later on. This wouldn’t be possible with immunofluorescence because the staining dissipates from the site of localization and loses its crispness over time. Several weeks or months after performing the initial immunofluorescent staining, that specimen can no longer be revisited for further imaging, meaning that the experiment would have to be repeated. This can be problematic for a number of reasons.
Another potential advantage of chromogenic methodology is that it gives you a real view into the underlying tissue architecture and morphology, whether that be adjacent blood vessels, cartilage, bone, etc. These structures might not be evident with immunofluorescence, and, unless you did an adjacent H&E stain, you’d only be seeing half the picture.
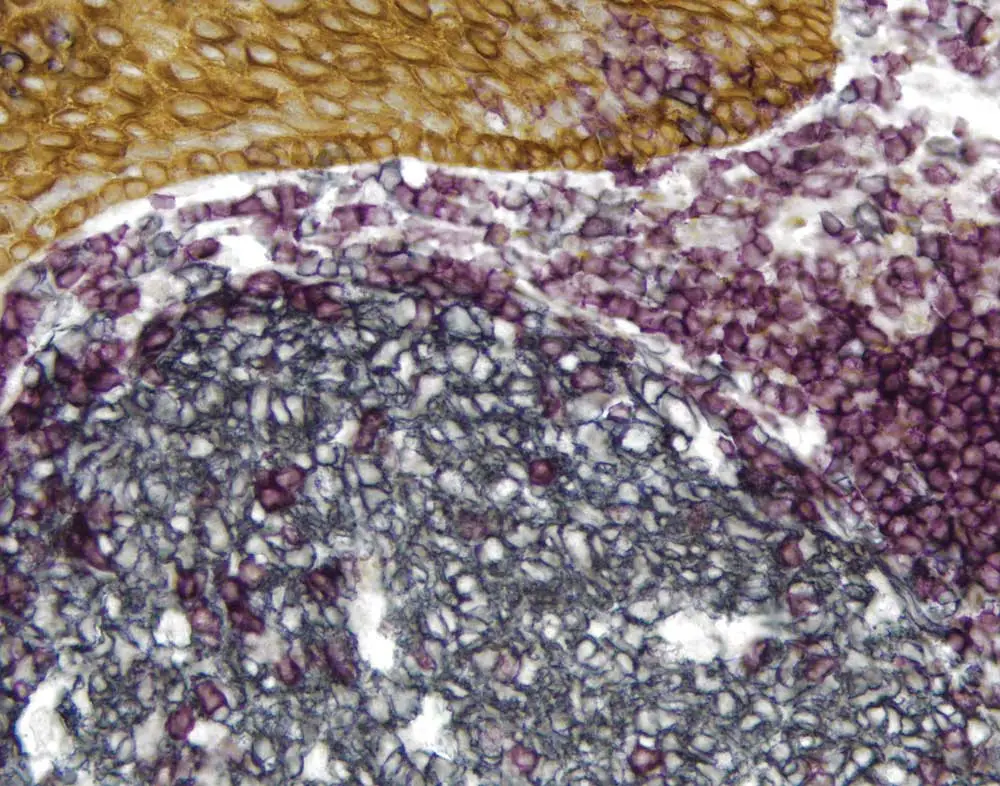
Chromogenic immunohistochemistry is also very accessible. Not everyone will have the filters and equipment required for fluorescent microscopy, but the chromogenic methodology is easy to get started with because it’s an extension of single label light microscopy. Moreover, immunofluorescence-based systems tend to take longer to set up and optimize.
Of course, that doesn’t mean that chromogenic multiplex immunohistochemistry is better than immunofluorescence — maybe archiving isn’t important to you, but you really want to look at multiple membrane markers, in which case you would likely choose immunofluorescence. The best system for you will depend on your application and experimental goals.
Yes. Primary antibodies can be directly conjugated with a peroxidase- or alkaline phosphatase–based enzyme, and multiple primary antibodies conjugated with an enzyme substrate can even be used together. An example of this would be one primary antibody conjugated to an anti-mouse HRP and the other to an anti-rabbit alkaline phosphatase primary antibody; these could be mixed together, and the substrates could then be reacted separately.
However, directly conjugated primary antibodies do tend to have relatively low sensitivity. If you are interested in a weakly expressed antigen or an antigen of unknown expression, such as in the case of upregulation, you may want to use a more sensitive detection methodology. Another great option might be a directly conjugated primary antibody in combination with an unconjugated second primary antibody followed by a different detection methodology, like an ImmPRESS polymer-based system.
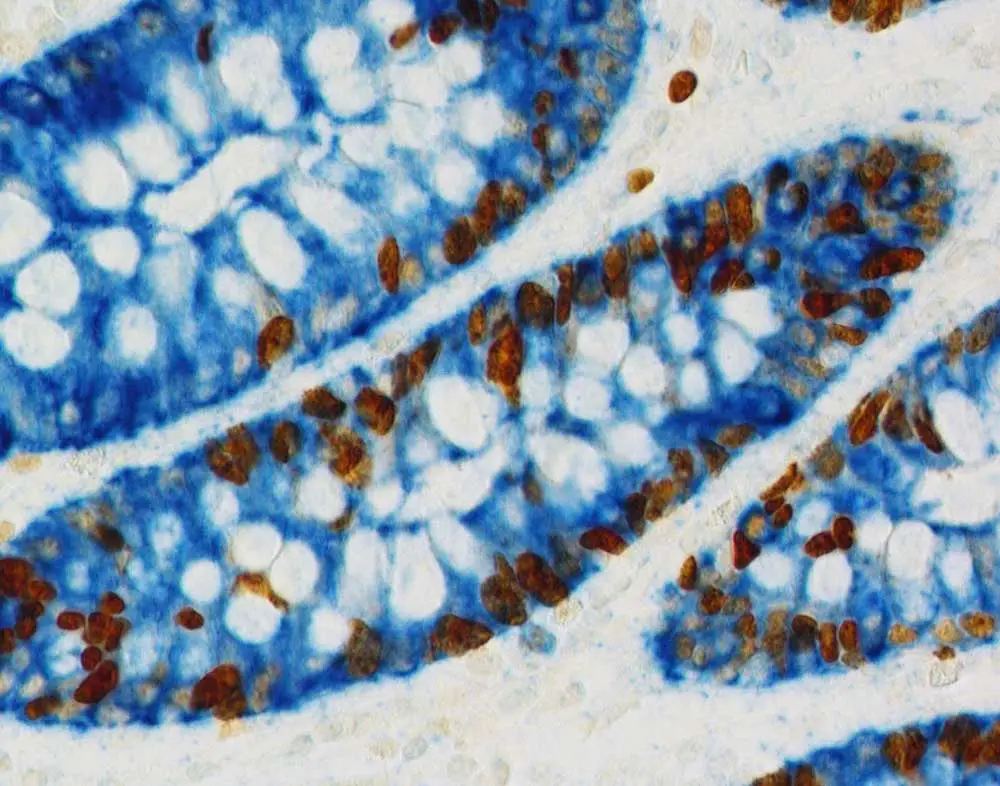
An example of this might be that for one primary antibody, a citrate at pH 6.0 is recommended for antigen retrieval, while the protocol for the second primary antibody suggests using an alkaline EDTA- or Tris-based reagent with a pH of 8.5 or 9.0. Typically, the reagent with the higher pH will be more likely to unmask multiple antigens.
With that said, if simply trying to do antigen retrieval on both antigens with the higher pH reagent doesn’t work, you can apply the reagents sequentially. In this situation, you might start with the citrate antigen retrieval reagent and go all the way through your workflow to color development using a heat-resistant substrate like DAB, which is resistant to unmasking. Then you would unmask your other antigen using the higher pH reagent and develop the second color.
If you’re using two primary antibodies raised in rabbit that target adjacent or different cellular structures within mouse tissue, the best method would be to apply them sequentially to avoid cross-reactivity. For example, you could detect the first rabbit primary antibody with peroxidase and develop with DAB, followed by application of the second rabbit primary antibody and either the same enzyme-based detection system plus a different colored substrate, or a different enzyme-based system with a different alkaline phosphatase– based substrate.
Enzyme substrates such as peroxidases and alkaline phosphatases precipitate out as solids at the site of localization. These substrates have unusual reaction kinetics, and if one is already present, another may not precipitate in the same location. All this means that multiple precipitating enzyme substrates don’t mix together and generate a third color in a reliable way.
Therefore, the chromogenic methodology probably will not work well to visualize CD3 and CD8 (both of which are membrane-based markers) in the same cell if you expect 100% overlap in their expression pattern. We would recommend a fluorescence-based system in this case. On the other hand, if you have two closely opposed or adjacent target antigens in the same cell, for example one membrane-based and one nuclear or cytosolic marker, you could certainly use a combination of peroxidase- or alkaline phosphatase–based substrates. DAB would be a great choice for the membrane marker because it gives a very nice, crisp demarcation, and an alkaline phosphatase could be used for the nuclear or cytosolic target.
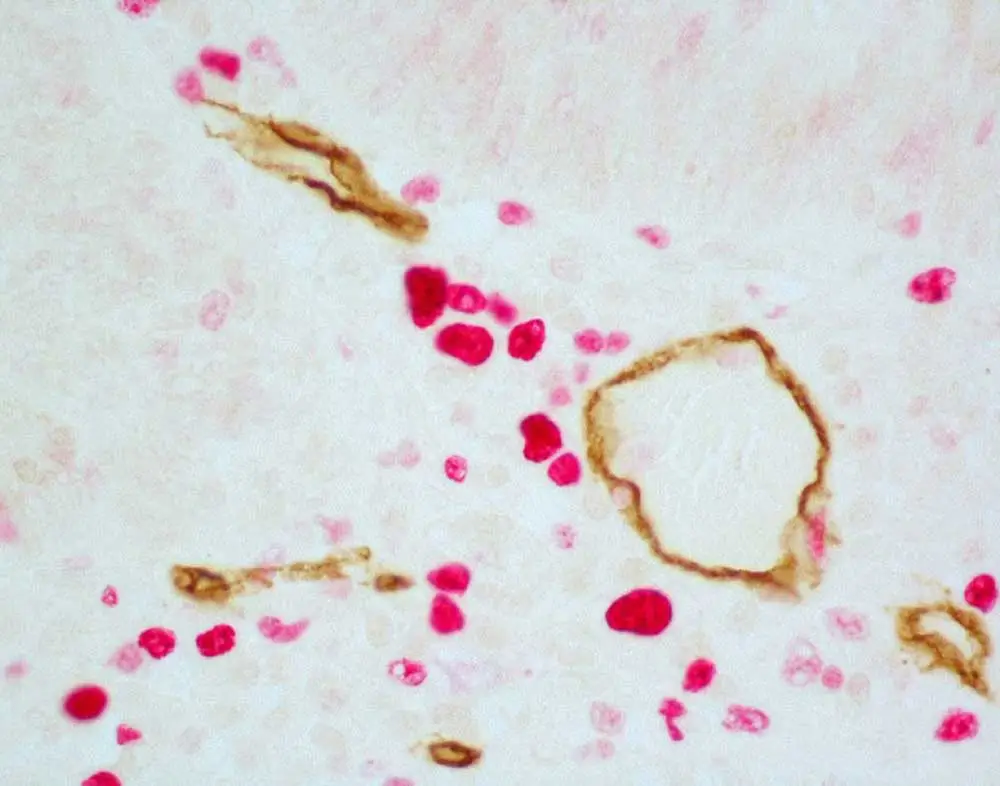
The ImmPRESS Duet Kit has a combination of anti-mouse secondary antibody and anti-rabbit secondary antibody plus your choice of either peroxidase or alkaline phosphatase. It’s a very easy methodology for double-labeling because it comes mixed in the same bottle as a pre-diluted, ready-to-use solution. The problem with applying the Duet Kit on rodent tissue is that the anti-mouse secondary antibody may cross-react with mouse or rat IgG present in the tissue, since these are commonly used lab rodent species. We don’t recommend the ImmPRESS Duet for experiments with rodent tissue because this cross-reactivity could generate false positive results.
An exception to this would be specimens that do not have any immunoglobulin present, for example a cell culture, in which case you could apply the ImmPRESS Duet Kit with no concerns of potential cross-reactivity. However, for most sections that have been excised from vascularized tissue, it’s important to keep the risk of cross-reactivity in mind.
If you still have questions, Vector Laboratories’ got answers! Watch the full multiplex immunohistochemistry webinar to supercharge your staining know-how, or download our IHC Multiplexing guide to help you get started.




Stay in the Loop. Join Our Online Community
Products
Ordering
About Us
Application
Resources

©Vector Laboratories, Inc. 2025 All Rights Reserved.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Privacy Statement