


Today, Courtney Young, PhD, is the CEO of gene therapy startup company MyoGene Bio. But back in 2008 when she was a senior in high school, her cousin was diagnosed with Duchenne muscular dystrophy (DMD). By that point in time, she already knew that she was interested in science, math, and research. But that diagnosis provided a strong motivation, giving her the focus she needed to pursue her future in science. As an undergrad at Johns Hopkins University, she began working in labs that studied DMD and realized that she enjoyed working on muscle biology and associated diseases. Since then, her interest has never faltered, and already she can claim 11 years of experience in the field of muscle research. Her cousin is now 15, and although he uses a wheelchair for long distances, he is otherwise happy and doing well, playing chess in tournaments. While he did participate in one clinical trial, it was focused on the side effects of the disease rather than the underlying cause. And though “there are a lot more clinical trials now than when he was first diagnosed, giving us hope,” Courtney has undertaken a direct role to make a difference.
“[…] some of the newer clinical trials with gene therapy, like what we’re developing, are targeting the underlying cause of the disease, and not just the side effects. We think this will have a much stronger impact on the disease progression. I pay attention to the clinical trials, not only for my work, but also for my family at the same time. Because I care more, I’ll spend more time and try harder to give good advice because it affects someone close to me.”
As one of three women co-founders and the CEO of MyoGene Bio (Los Angeles, CA), incorporated in 2018, she took on a full-time role in 2019, just after completing her postdoctoral research. These days, she spends about a third of her time in the lab doing benchwork, such as immunostaining and imaging, and the remaining balance on research management and the business of doing business. Of course, when you work at a startup or company with just a few employees, you need to wear many hats. Courtney Young knows this well. She works on the finances of the organization in collaboration with an accountant, legal aspects including patent submissions with a lawyer, and business decisions with her co-founders—trusted scientific advisors in both her graduate life and now in this entrepreneurial adventure—while managing full-time and part-time employees (two of each), applying for and juggling administration of three grants, building relationships to foster the business and garner investors, and so much more. The “more” part includes a few things that were a surprise to her: handling the bulk of the company administrative tasks and writing, signing, and mailing out checks to pay the bills. Despite all these smaller, seemingly mundane tasks, Courtney knows they are essential steps to build the business and meet their current goals and future aspirations.
Founded in 2018, the mission of MyoGene Bio is “to develop cutting edge genetic therapies for muscle diseases,” and for Courtney, this initiative continues to be personal, with the hope of someday being able to help her cousin who suffers from DMD, the most common form of muscular dystrophy. Rooted in a deficiency of the dystrophin protein from a genetic mutation in the DMD gene on the X chromosome, this muscle disease primarily affects boys and their body’s ability to form and maintain healthy muscle. Although she has always had an interest in math and science, especially biology, her cousin’s diagnosis was a formative touchstone for her career pathway. By 2012, she had earned a BS in Chemical and Biomolecular Engineering (Johns Hopkins University, Baltimore, MD), worked in three labs studying muscular dystrophy, and interned at Covidien (now Medtronic, Boulder, CO) and Sarepta Therapeutics (Bothwell, WA) focusing on tissue engineering and biology discovery research, respectively. In 2013, she added a Master of Research in Biomedicine from University College London (UK), before beginning a long-term association with University of California, Los Angeles (UCLA; Los Angeles, CA), to pursue a PhD in Molecular Biology and complete a postdoctoral rotation in Neurology.
“As we were getting ready to publish the first paper related to my thesis, my advisors and I thought that we should tell UCLA about our potential gene therapy invention. Although they initially were not sure that they wanted to patent it, they changed their mind right before publication. That decision got us to thinking that if it’s worth patenting, then we have a proprietary gene therapy approach that we could build a company around and develop it. We realized that we should be the ones to develop it and do a startup, and we did just that. Although UCLA owns the patent, we’re listed as the inventors, and MyoGene has licensed the intellectual property so we can use it.”
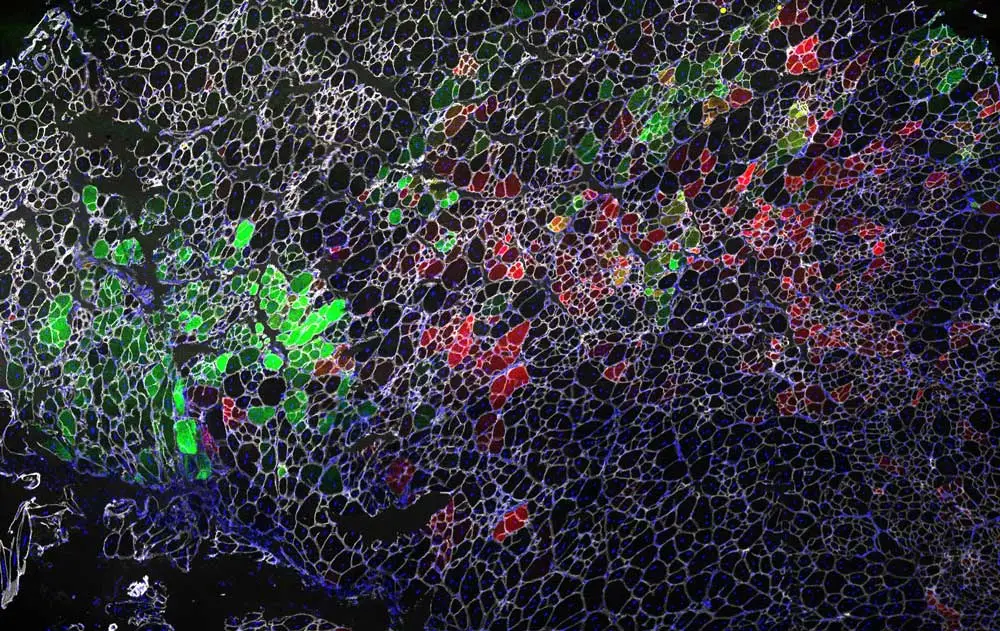
Her years at UCLA included the development of the gene editing platform that forms the basis of the gene therapy her company calls MyoDys45-55. This gene therapy targets the hotspot location where half of the mutations in DMD occur, and where a deletion mutation (exon 45-55) is associated with some of the mildest symptoms in Becker muscular dystrophy patients, who can remain asymptomatic into their 60s. Gene editing is an attractive dystrophin-restoration strategy because it offers a permanent solution for slowing or stabilizing disease progression, modifying the problematic areas of the patient’s own DNA sequence without affecting the rest of the gene. The preclinical development underway at MyoGene Bio relies on CRISPR-Cas9, the groundbreaking gene editing technology first patented in 2012 and awarded the Nobel Prize in Chemistry in 2020. At present, MyoDys45-55 has been demonstrated to restore dystrophin via CRISPR/Cas9 gene editing in human stem cells and in mice.
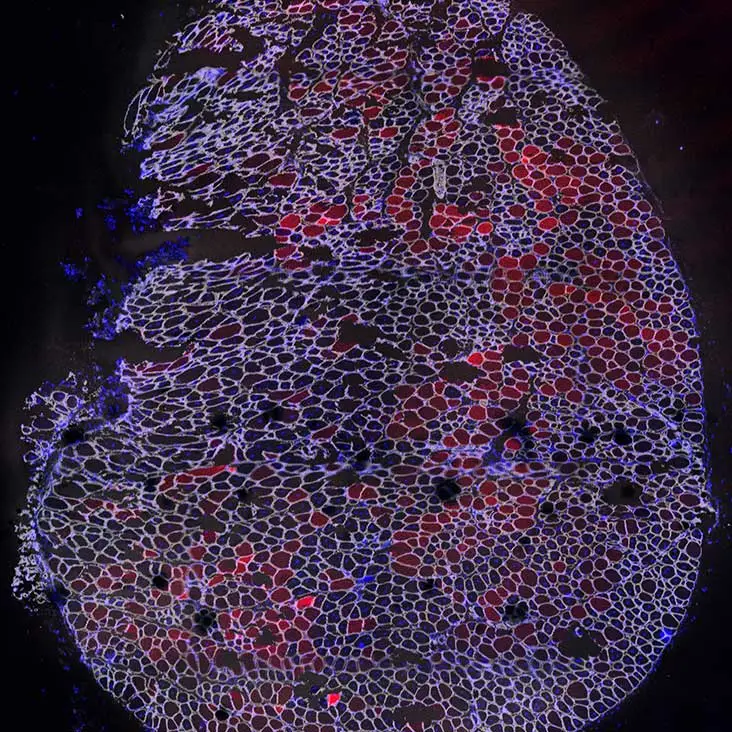
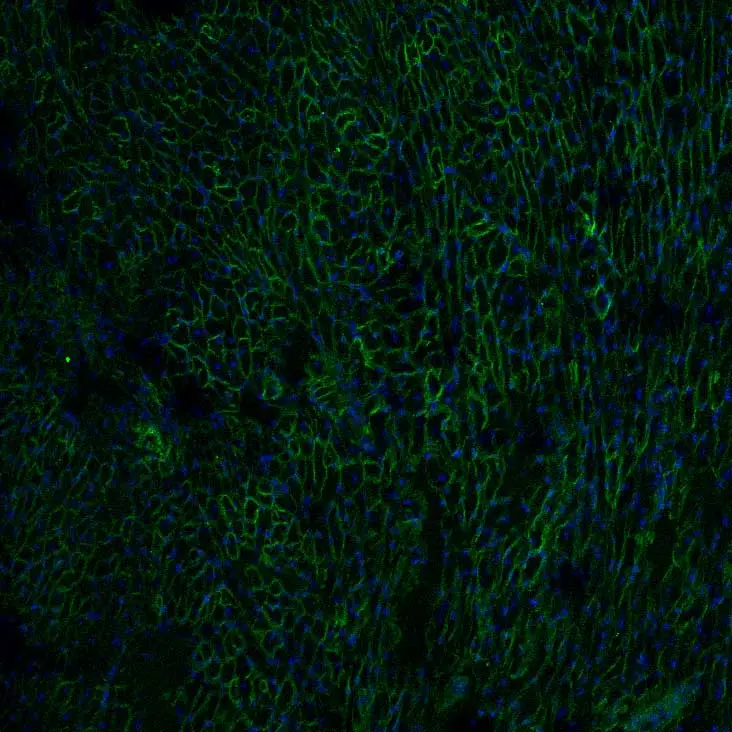
“Looking at muscle under the microscope is really neat. I typically view muscle sections from treated mice, using the Vector Lab M.O.M. Basic Kit and VECTASHIELD Antifade Mounting Medium with DAPI. You see so many tightly packed fibers and a honeycomb structure when you cut it cross-sectionally. While the images are abstract, pretty, and colorful, I end up thinking even more about the scientific story behind the images, like seeing a protein that wasn’t there before or two colors where normally in DMD I would only see one.”
Courtney understands that delivery of CRISPR technology into muscle will be challenging because it will need to be delivered to all the muscles throughout the body, including diaphragm and cardiac muscle. At present, the adeno-associated viral delivery system they’re using works, but she recognizes that it isn’t ideal—the system has immune response issues, requires usage of high doses, is costly to manufacture, and, ultimately, may need an upgrade. However, she knows that the return on her investment will be well worth the effort as gene editing has the potential to affect disease progression and improve quality of life for patients struggling with muscle diseases. In a few years or so, she hopes that they’ll be able to move their first therapy into early-stage clinical trials. After that, the goal is to find a partner company to manage the larger scale clinical trial and commercialization aspects. This approach would allow the company to continue focusing on R&D, which is where Courtney’s heart is.




Stay in the Loop. Join Our Online Community
Products
Ordering
About Us
Application
Resources

©Vector Laboratories, Inc. 2025 All Rights Reserved.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Privacy Statement