Immunohistochemistry (IHC)
Over 40 years ago, Vector Laboratories revolutionized the field of IHC with the commercialization of antibody-based avidin-biotin reagents and the introduction of the VECTASTAIN® ABC system. Since then, we have worked tirelessly to improve the accuracy, sensitivity, and reproducibility of IHC, continually expanding our portfolio with innovative products designed to support every step of the IHC workflow.
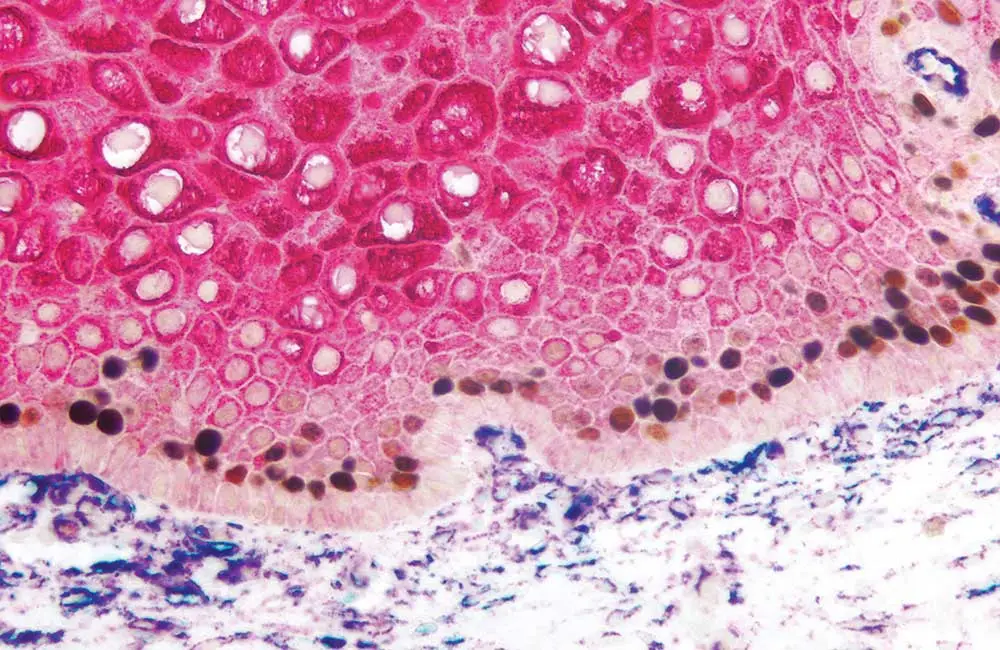
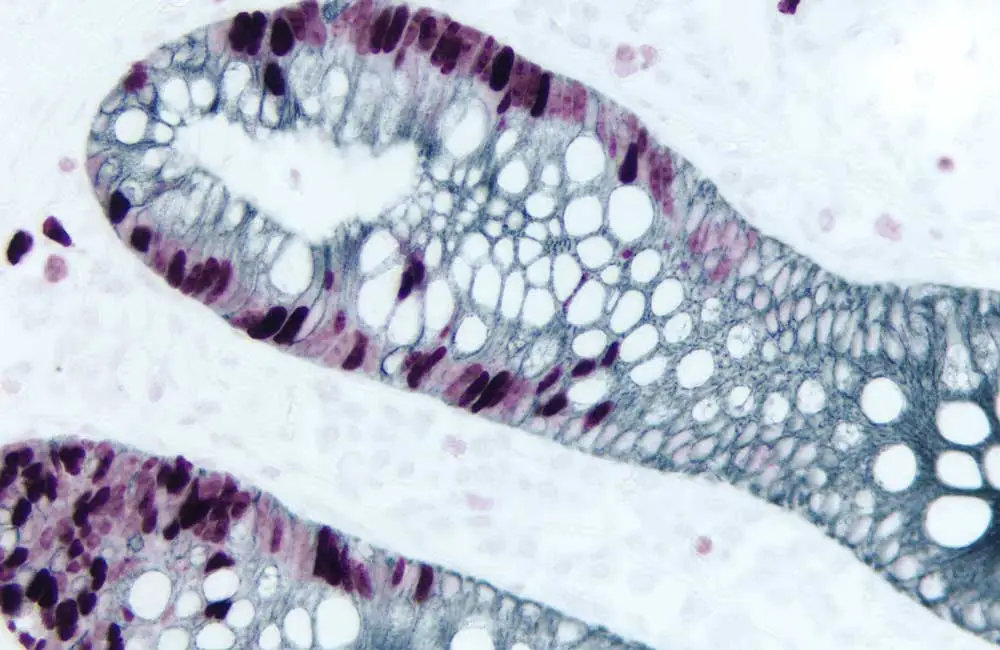
What is immunohistochemistry?
IHC is an important, microscopy-based technique used to visualize the distribution and abundance of target biomolecules within a tissue sample. It relies on the exquisite specificity and sensitivity of antibodies to detect cellular components of interest and reveals valuable information about target expression under conditions of both health and disease. A major advantage of IHC over other immunoassay techniques such as Western blot, ELISA, and flow cytometry is that it preserves the natural tissue architecture. This allows for the study of complex intercellular interactions – a feat that is impossible when samples must be lyzed for analysis.
Why is immunohistochemistry important?
Because IHC allows researchers to see exactly where defined targets are expressed – in a given tissue sample, it is widely used to support a broad range of applications. These include the study of normal physiological processes like tissue and organ development, wound healing, and the immune response, as well as the multitude of cellular events that contribute to disease pathology. IHC is also routinely employed for diagnostic purposes. For example, IHC can be used to determine the stage and grade of a tumor, to identify the source of a tumor metastasis, or to evaluate the effects of arthritis.
How is immunohistochemistry performed?
IHC follows a sequential protocol that begins with sample collection and ends with visualization of tissue sections using a microscope. Although the individual IHC protocol steps can vary depending on the tissue type and the target of interest, they generally involve:
- Tissue collection for immunohistochemistry (IHC)
- Immunohistochemistry (IHC) fixation
- Immunohistochemistry (IHC) tissue embedding
- Immunohistochemistry (IHC) sectioning and application to glass slide
- Immunohistochemistry (IHC) deparaffinization and rehydration
- Immunohistochemistry (IHC) antigen retrieval
- Immunohistochemistry (IHC) protein blocking
- Blocking of endogenous enzymes for immunohistochemistry (IHC)
- Immunohistochemistry (IHC) immunodetection
- Immunohistochemistry (IHC) counterstaining
- Immunohistochemistry (IHC) mounting / coverslipping
Many stages of the IHC workflow can be automated. However, when it comes to immunodetection, a manual staining method offers greater flexibility than automation and allows researchers to thoroughly optimize conditions for a specific antibody-antigen reaction.
Tissue collection for immunohistochemistry (IHC)
The method used to collect and preserve tissue samples for IHC is largely dictated by the tissue type and the target of interest. In general, excised human and animal biopsy material is either snap-frozen immediately upon collection (using isopentane mixed with dry ice) or immersed in a suitable fixative solution prior to further downstream processing. Whichever method is chosen, it is important to work quickly to avoid compromising the integrity of sample material and generating misleading IHC results.
Immunohistochemistry (IHC) fixation
Tissue fixation functions to preserve tissue and cell morphology and maintain the antigenicity of target molecules for IHC. The choice of fixative solution, duration of tissue fixation, and the tissue-to-fixative ratio can all affect the extent and intensity of IHC staining.
Formalin (formaldehyde) is the most widely used fixative solution for IHC. For most excised tissues, it is recommended that formalin fixation be performed for 24 hours at room temperature, using a tissue to fixative ratio of 1:1 to 1:20. Longer fixation times should be avoided to prevent irreversible epitope masking that results from formalin cross-linking proteins. Where formalin fixation is included in IHC workflows, antigen retrieval is usually necessary for epitopes to be made accessible for antibody binding.
Alternative fixative solutions for IHC include acetone, and alcohols such as ethanol or methanol. The latter are more commonly used during IHC analysis of nucleic acids and post-translational modifications. With alcohol fixation, antigen retrieval is not required, but a drawback of alcohol fixatives is that they can distort nuclear and cytoplasmic features and can lead to tissue shrinkage during long term storage.
Immunohistochemistry (IHC) tissue embedding
Tissue embedding functions to preserve sample structure as well as facilitating IHC tissue sectioning. Formalin-fixed tissue samples are typically dehydrated by submersion in increasing concentrations of alcohol before being embedded in paraffin wax – hence the term FFPE for formalin-fixed paraffin-embedded tissue. They can then be stored at room temperature. Frozen tissue samples for IHC are instead encased in a specialized cryo-embedding medium such as optimal cutting temperature compound (OCT) and stored at -80°C. For frozen tissues, fixation usually takes place after sectioning.
Immunohistochemistry (IHC) sectioning and application to glass slide
For tissue samples to be processed for IHC and visualized using a microscope, they must be cut into sections of approximately 4-6 µm thickness. For FFPE tissues, this is achieved using a microtome, whereas for frozen tissues a pre-cooled cryostat is required. Sections are then mounted on to glass slides that have been coated with a specialized tissue section adhesive and clearly labeled with a solvent-resistant pen. For optimal results, IHC should always be performed using freshly cut sections.
Once mounted, FFPE sections are dried in an oven, whilst frozen sections may be dried at room temperature prior to fixation. Before proceeding with the IHC workflow, it is common practice to use a hydrophobic barrier pen to draw around the tissue section. This creates a heat-stable, water-repellent barrier for keeping IHC reagents localized on tissue specimens, or to prevent reagent mixing when multiple sections are mounted on the same slide.
Immunohistochemistry (IHC) deparaffinization and rehydration
Where tissues are paraffin-embedded, it is essential that they are deparaffinized prior to IHC immunostaining since any paraffin present will prevent antibody binding. They must then be rehydrated before being progressed through the IHC workflow. Deparaffinization is usually performed by immersing slides in xylene, whereas rehydration involves a series of incubations in decreasing concentrations of alcohol.
Immunohistochemistry (IHC) antigen retrieval
Antigen retrieval is an essential step in most IHC workflows using FFPE tissue samples. It functions to revert formalin cross-linking, allowing antibody reagents access to epitopes for binding, and must be carefully optimized for each antibody-antigen pair.
The most widely used method for IHC antigen retrieval involves immersing the deparaffinized tissue sections in a citrate- or Tris-based buffer before heating with a microwave, pressure cooker, autoclave, or water bath. During heat-induced antigen retrieval, it is critical to optimize the incubation temperature and duration, and the pH of the antigen retrieval solution.
Another approach to IHC antigen retrieval is to use enzymes such as proteinase K, trypsin, or pepsin to unmask epitopes. This is achieved by enzyme digestion of the protein cross-links. For enzyme-induced antigen retrieval, temperature, incubation time, and enzyme concentration are all key factors requiring optimization.
Immunohistochemistry (IHC) protein blocking
Most immunoassays include a protein blocking step to prevent the occurrence of unwanted background signal, and IHC is no exception. Background signal occurs mainly as a result of antibody reagents binding non-specifically to proteins other than their target antigens, making it essential to block any potential binding sites with a carefully chosen blocking buffer.
For IHC, it is often advised that 1–10% normal serum from the same species as the secondary antibody host is used for blocking. Other widely used blocking buffers for IHC are BSA, casein, and designated animal-free buffers intended for use in multiplex IHC applications where antibodies from several different species will be combined.
Additional specialized blockers include glycoprotein-free blocking agents for IHC applications where glycoprotein contamination could generate background staining or false positive results, and reagents that enable primary antibodies raised in a mouse host to be used for IHC staining of mouse tissue samples. Because mouse tissue contains endogenous mouse immunoglobulins, it is important to prevent anti-mouse antibodies from binding these by including a mouse-on-mouse blocking step in IHC staining protocols.
Blocking of endogenous enzymes for immunohistochemistry (IHC)
With many IHC workflows relying on the use of antibodies labeled with horseradish peroxidase (HRP) or alkaline phosphatase (AP) for detection, it is of paramount importance to block endogenous enzyme activity in tissue samples. Endogenous peroxidase activity is commonly blocked using 3% hydrogen peroxide, whilst 10mM levamisole is frequently used to block endogenous alkaline phosphatase. In many cases, a more convenient approach is to use a dual enzyme blocking reagent able to block both peroxidase and alkaline phosphatase activity simultaneously since this can increase the efficiency of IHC workflows. Endogenous biotin, biotin receptors, and avidin or streptavidin binding sites can also be sources of unwanted background signal when processing tissues for IHC. These can be blocked using an avidin/biotin blocking kit or streptavidin/biotin blocking kit depending on the nature of the IHC detection system that is chosen.
Immunohistochemistry (IHC) immunodetection
It is inarguably the immunodetection step of any IHC workflow where researchers face the greatest choice. This ranges from the selection of a suitable antibody all the way through to deciding on an appropriate IHC detection system.
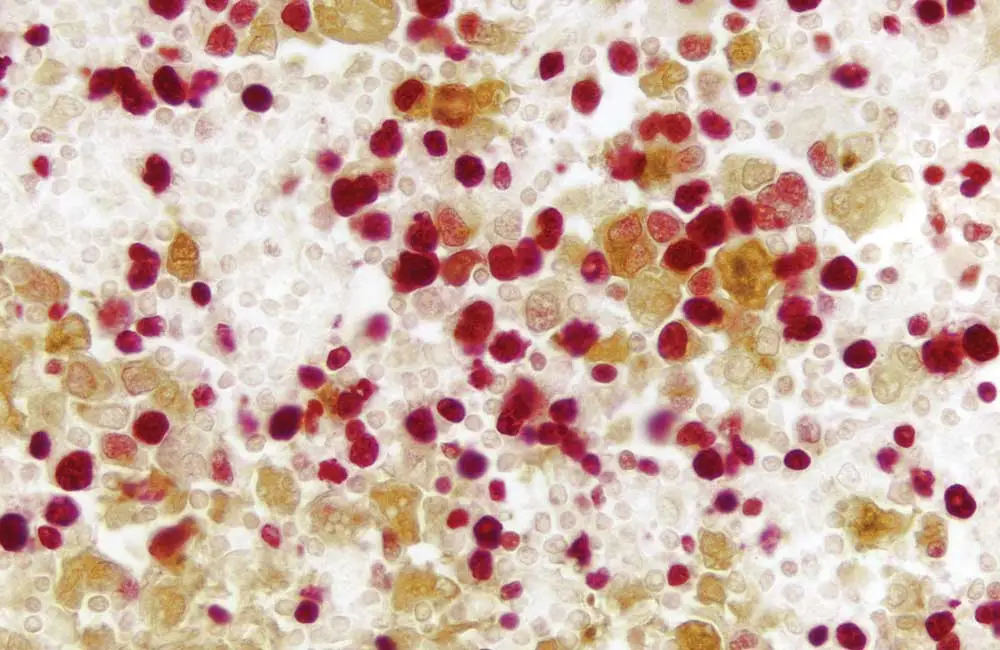
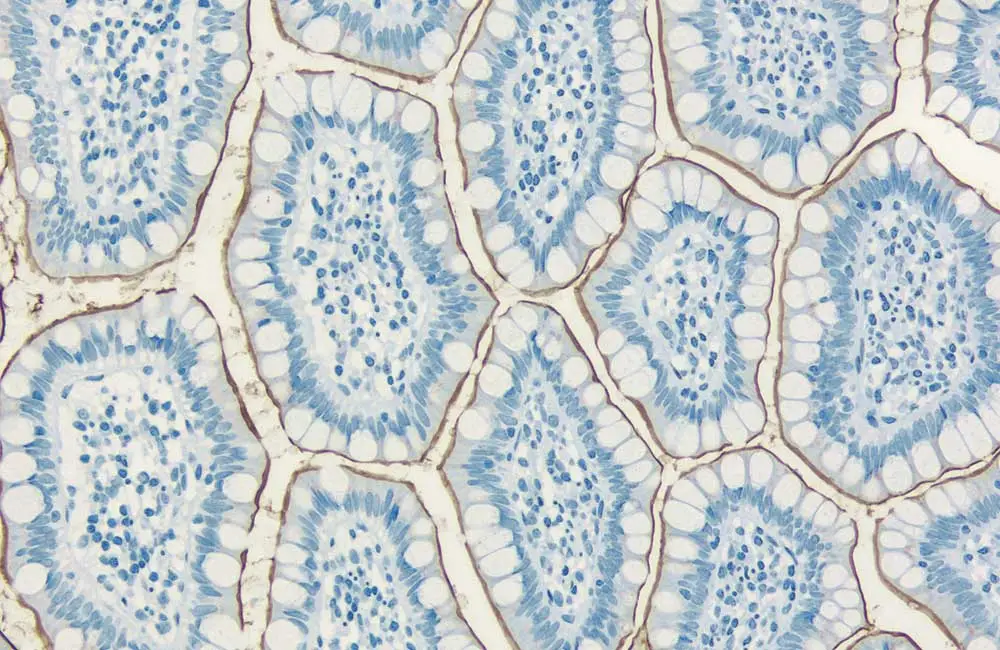
Antibodies can broadly be divided into two distinct categories, namely polyclonal and monoclonal antibody reagents. Whilst polyclonal antibodies exist as a heterogenous population, able to recognize many different epitopes, monoclonal antibodies are identical and all bind the same epitope. An advantage of using polyclonals for IHC is that they may provide improved sensitivity through multi-epitope recognition, however they are available in only finite amounts and produce less consistent results due to batch-to-batch variability. In contrast, monoclonals can offer greater specificity and reproducibility, as well as being available in unlimited supply.
Whichever antibody format is chosen, it is vital to confirm it has been thoroughly validated for IHC applications. Direct or indirect detection is another important decision. During direct detection, the primary antibody is labeled, whereas indirect detection involves the use of a labeled secondary antibody. HRP- or AP- conjugated secondary antibodies are often used for IHC, where they are paired with dedicated HRP substrates and AP substrates for colorimetric detection. The choice of HRP or AP is driven mainly by antigen abundance and the level of endogenous enzyme present in the tissue section; HRP substrates such as 3,3′-diaminobenzidine (DAB) generate an optically dense product and are widely used for IHC, whereas AP substrates produce more translucent precipitates that can be better suited to visualizing broadly expressed targets or the underlying tissue morphology.
Biotinylated secondary antibodies are also extremely popular for IHC and are routinely used in combination with enzyme-conjugated avidin or streptavidin or a VECTASTAIN® ABC kit. By complexing both avidin and biotin with either HRP or AP, VECTASTAIN® ABC kits exploit the high-affinity avidin-biotin interaction for reliable, extremely sensitive IHC detection of any biotinylated molecule.
Where antigenic targets are of low abundance and a stronger IHC signal with low background is required, enzyme polymer detection systems represent an effective solution. Based on an inert molecule able to bind both antibodies and enzymes, these reagents introduce a high concentration of enzyme at the site of specific antigen localization for an enhanced signal: noise ratio.
Immunohistochemistry (IHC) counterstaining
After slides have been stained for the target of interest, it is often necessary to add a counterstain. By identifying key features such as nucleic acids, cytoplasm, or discrete cellular compartments, counterstains are helpful to put primary IHC staining into context. Hematoxylin, Methyl Green, and Nuclear Fast Red are all examples of different colored counterstains for nuclei, and can readily be combined with eosin for cytoplasmic IHC staining.
Counterstain / Substrate Compatibility
This table is designed as a reference to determine the optimal counterstain / substrate combination for your application. Consideration should be given to tissue type, antigen unmasking protocol, and other detection parameters to achieve the desired staining intensity.
| Substrate | Catalog Number | Hematoxylin (H-3404) Hematoxylin QS (H-3401) |
Methyl Green (H-3402) | Nuclear Fast Red (H-3404) |
|
ImmPACT DAB (brown) |
SK-4105 | Excellent Contrast | Excellent Contrast | Fair Contrast |
|
ImmPACT DAB EqV (brown) |
SK-4103 | Excellent Contrast | Excellent Contrast | Fair Contrast |
|
DAB (brown) |
SK-4100 | Excellent Contrast | Excellent Contrast | Fair Contrast |
|
DAB-Ni (gray-black) |
SK-4100 | Excellent Contrast | Fair Contrast* | Good Contrast |
|
ImmPACT AEC (red) |
SK-4205 | Excellent Contrast | Counterstain Incompatibility** | Color Incompatibility |
|
ImmPACT AMEC Red (red) |
SK-4285 | Excellent Contrast | Counterstain Incompatibility** | Color Incompatibility |
|
AEC (red) |
SK-4200 | Excellent Contrast | Counterstain Incompatibility** | Color Incompatibility |
|
TMB (blue) |
SK-4400 | Color Incompatibility | Counterstain Incompatibility | Excellent Contrast |
|
ImmPACT VIP (purple) |
SK-4605 | Fair Contrast | Excellent Contrast | Poor Contrast |
|
Vector VIP (purple) |
SK-4600 | Fair Contrast | Excellent Contrast | Poor Contrast |
|
ImmPACT SG (blue-gray) |
SK-4705 | Poor Contrast | Good Contrast | Excellent Contrast |
|
SG (blue-gray) |
SK-4700 | Poor Contrast | Good Contrast | Excellent Contrast |
|
ImmPACT NovaRED (red) |
SK-4805 | Excellent Contrast | Excellent Contrast*** | Color Incompatibility |
|
Vector NovaRED (red) |
SK-4800 | Excellent Contrast | Excellent Contrast*** | Color Incompatibility |
|
ImmPACT Vector Red (magenta) |
SK-5105 | Excellent Contrast | Excellent Contrast | Color Incompatibility |
|
Vector Red (magenta) |
SK-5100 | Excellent Contrast | Excellent Contrast | Color Incompatibility |
|
Vector Black (black) |
SK-5200 | Excellent Contrast | Excellent Contrast* | Excellent Contrast |
|
Vector Blue (blue) |
SK-5300 | Color Incompatibility | Good Contrast | Excellent Contrast |
|
BCIP / NBT (indigo) |
SK-5400 | Color Incompatibility | Excellent Contrast* | Excellent Contrast |
|
*This substrate shows a slight decrease in sensitivity following the methyl green protocol. This decrease can be minimized by reducing the heat incubation and acetone rinse times. |
||||
Immunohistochemistry (IHC) mounting / coverslipping
Mounting represents the final stage in the IHC workflow, whereby a cover slip is added to the slide to protect the immunostained sample. Cover slip attachment typically involves the use of a specialized mounting medium designed to permanently preserve IHC stains or precipitable enzyme substrates. The selection of an appropriate mounting medium will be dictated by the IHC detection method.
The success of any IHC application depends on selecting the right reagents. By developing innovative products to enhance every step of the IHC workflow, Vector Laboratories is here to help. Contact us to find out more.




