FAQs
- Immunohistochemistry
- Immunofluorescence
- Lectin Glycobiology
- Bioconjugation
- dPEG®
- Immunohistochemistry
- General Questions
- VectaMount Express
- VECTASTAIN ABC Kits
- ImmPRESS Polymer Reagents
- Enzyme Substrates
- Blocking Reagents
- ImmEdge Hydrophobic Barrier Pen
Advice? I’m new to immunohistochemistry, do you have any recommendations for how best to get started?
Yes we do. Please visit our comprehensive Immunohistochemistry (IHC) page for assistance with each step of the IHC workflow. This resource contains a great deal of technical material including product recommendations and links to other technical documentation / videos / webinars.
After mounting with VectaMount Express, why do my slides look cloudy?
It is important to use 99+% alcohol (isopropanol or ethanol) for the brief dehydration steps. Lower alcohol grades will not be viable substitutes. Slides should be rinsed in tap water (not buffer) prior to dehydration, as salts in buffers can precipitate if not removed. Depending on slide volume, alcohol baths should be changed out on a regular basis with fresh solution. It is suggested to drain excess water when transferring slides from tap water into the alcohol bath.
Can I apply the ImmEdge Pen residue to wet slides?
Yes. The ImmEdge Pen residue contains a wax constituent that allows for application to microscope slides that have buffer or similar aqueous solutions on the surface. Applying the pen residue to wet slides does not affect adhesion of the residue nor interfere with the tissue section.
Can I dry the Vectamount Express mounted slides in an oven?
No. It is recommended to dry slides at ambient room temperature.
Can I perform double staining for two different antigens on the same section using two mouse primary antibodies with the same VECTASTAIN ABC Mouse IgG Kit?
Yes. We do offer complete protocols, helpful tips as well as enzyme substrate combination suggestions and images on our website for this application at the following link: https://go.vectorlabs.com/multiplexing. Essentially the staining is performed sequentially (one stain after the other) from primary antibody right through to substrate color development using two different enzyme substrates.
Can I use the same ImmPRESS anti-mouse IgG polymer reagent (MP-7402) to perform double labeling with two different mouse primary antibodies on the same tissue section?
Yes, the same ImmPRESS peroxidase secondary detection reagent can be used for double staining. There are a few considerations. First, we would suggest optimizing each single stain first on different tissue sections. Once the conditions for each stain have been established, the single assays should be run sequentially, from primary antibody though substrate color development, to achieve double staining on the same section. Secondly, different peroxidase substrates will have to be used to provide adequate color contrast and reliable target antigen localization. This double staining approach is most reproducible when detecting antigens expressed in different cell types on the same section, or different cell compartments of the same cell type. Please see our multiple antigen labeling guide for additional considerations:
Can I use the same ImmPRESS anti-mouse IgG polymer reagent (MP-7402) to perform double labeling with two different mouse primary antibodies on the same tissue section?
Yes, the same ImmPRESS peroxidase secondary detection reagent can be used for double staining. There are a few considerations. First, we would suggest optimizing each single stain first on different tissue sections. Once the conditions for each stain have been established, the single assays should be run sequentially, from primary antibody though substrate color development, to achieve double staining on the same section. Secondly, different peroxidase substrates will have to be used to provide adequate color contrast and reliable target antigen localization. This double staining approach is most reproducible when detecting antigens expressed in different cell types on the same section, or different cell compartments of the same cell type. Please see our multiple antigen labeling guide for additional considerations:
Could you please describe what would be an appropriate negative control for IHC that can be used to help validate tissue staining results?
A commonly used negative control is omission of the primary antibody. While this control addresses whether the secondary antibody reagents are a source of staining, inadvertent binding of the primary antibody to the tissue can occur. Various tissue elements such as Fc receptors and charged molecules may bind the primary antibody non-specifically. Simply omitting the primary antibody as a negative control would miss potential false positive staining by this means. A few “publication worthy” negative controls for IHC are listed below: 1) Preabsorption of primary antibody with the immunogen used to generate the antibody can be employed. The working dilution of the primary antibody and an optimized concentration of the immunogen are incubated together for a period prior to application to the specimen. Lack of staining would indicate specificity of the primary antibody to the target antigen in solution. Positive staining using this method, however, may indicate lack of specificity of the primary antibody and/or the primary antibody is being bound by tissue elements. To rule out the latter, this control can be used in combination with suggestion no. 2 below. Note that adsorption controls are not always feasible or practical depending on the cost or source of the immunogen. 2) Use of an isotype control (e.g. “non-immune” mouse IgG), matched to that of the primary antibody and applied at the same protein concentration as the primary antibody, is probably the most widely used negative control. This control addresses whether tissue elements are inadvertently binding immunoglobulin from the same species as the primary antibody, in addition to non-specific binding from the secondary detection reagents. In most cases, use of a sub-class of isotype immunoglobulin (e.g. mouse IgG2a or IgG2b) is not required. Note that the use of pre-immune immunoglobulin, obtained prior to immunization, could also be used. However, it is very unusual for commercial vendors to offer pre-immune immunoglobulin. 3) Substitution of the primary antibody with an “irrelevant antibody” is also a suitable negative control. The term “irrelevant” refers to a primary antibody of the same isotype as the specific primary antibody (i.e. mouse IgG) and applied at the same concentration, that is known not to bind to a target in the tissue specimen. An example would be an anti-cytokeratin antibody on smooth muscle tissue. As with negative control no. 1 above, lack of staining indicates tissue elements are not binding this isotype of immunoglobulin. 4) In some cases, the target antigen can be removed from the tissue specimen as a sort of “knock-out” preparation. Once the target antigen has been removed, the complete assay is run to determine lack of staining. One method to remove the target antigen is using defined enzyme digestion. Examples include the use of a collagenase if the target antigen is collagen, or hyaluronidase if the target antigen is hyaluronic acid. Of course, there are limitations to this approach, however, variations of this “deletion” or “knock-out” approach would be valid negative controls.
Do I have to apply the avidin/biotin blocking kit reagents before the primary antibody?
If the primary antibody is unconjugated, then the avidin/biotin blocking kit reagents can be applied at any time prior to the application of the biotinylated secondary antibody. If the primary antibody is biotinylated, then we would recommend applying the avidin/biotin blocking kit reagents before primary antibody incubation.
Does BLOXALL quench intestinal alkaline phosphatase activity?
Yes. BLOXALL quenches endogenous peroxidase enzyme activity in addition to all alkaline phosphatase (AP) isoforms including intestinal AP.
Does the ImmEdge Pen residue fluoresce?
Yes, the pen residue does fluoresce. However, this property does not limit the pens use to only light microscope applications. We routinely use the ImmEdge pen for immunofluorescent tissue staining applications. Usually the pen residue is applied well outside of the tissue section perimeter, and as such, the inherent fluorescent properties of the pen residue do not interfere with specific fluorescent signal.
Does VECTASHIELD PLUS perform equivalently or better with Alexa Fluor dyes compared with the regular VECTASHIELD (H-1000 & H-1200)?
Yes, the antifade mounting media are at least equivalent in signal retention. We have not seen any reduction in performance with any fluorophores evaluated, and with some dyes (i.e. Alexa Fluor 647 and Dylight 649) there are advantages.
For my IHC application, I intend to apply a mouse IgG primary antibody on rat tissue sections. Should I use the Vector Mouse on Mouse detection kit?
Vector Laboratories’ Mouse on Mouse detection kits (M.O.M.®) are designed for the detection of mouse IgG primary antibodies on mouse tissue sections. For detecting mouse primary antibodies on rat tissue, we offer a selection of anti-mouse, rat adsorbed secondary detection reagents. These are intended to be used for this application and would generate the most optimal signal to noise staining ratio. We offer a biotinylated anti-mouse IgG, rat adsorbed secondary antibody (Cat. No. BA-2001) for use with VECTASTAIN® ABC kits or avidin and streptavidin enzyme conjugates. Alternatively, we offer an ImmPRESS® HRP polymer anti-mouse IgG, rat adsorbed detection kit (Cat. No. MP-7422) for a convenient, one-step IHC methodology.
How can I successfully remove the coverslip once it has been applied to a slide with VectaMount Express Mounting Medium?
Place the slides in a glass staining rack or Coplin jar and completely submerge the slides in xylene overnight. After this time the coverslip should be easy to remove from the slide.
How do I choose which VECTASTAIN ABC Kit to use?
We offer a choice of either peroxidase or alkaline phosphatase based VECTASTAIN ABC kit detection systems. Selection of which enzyme system to use would be the first step. Most IHC applications involve detecting an unconjugated primary antibody. Consider the species in which the primary antibody is raised (e.g. rabbit), and then match to to corresponding species-specific VECTASTAIN ABC kit (e.g. VECTASTAIN ABC Rabbit IgG kit). If you already have a biotinylated target (i.e. biotinylated secondary antibody or primary antibody), then a standard VECTASTAIN ABC kit containing just the avidin/biotinylated enzyme reagents would be required. Note that only the VECTASTAIN Universal Elite PLUS ABC kit (PK-8200) contains a substrate.
How do I use PBS or TBS buffer to remount my slide using VectaMount AQ Aqueous Mounting Medium?
We would suggest using a Coplin jar or slide rack to completely submerge the slides/coverslipped sections in PBS or TBS buffer. Place the slides on their side or on their end and not lying flat. We would suggest leaving them submerged for an extended period, which may be overnight or longer (48-72 hours).
How long can I store slides mounted with the VECTASHIELD Vibrance?
After mounting with VECTASHIELD Vibrance, slides can be stored at room temperature for several months without media retraction, bubble formation, or loss of signal intensity. We have not seen any difference between room temperature or storage at 4 degrees C.
How long can the slides remain in isopropanol before mounting with Vectamount Express?
There are no specific time limits for slides to be in the alcohol bath. Depending on the number of slides in a given assay, the last slide to be mounted will be in the alcohol bath longer than the first mounted slide. This may take several minutes or much longer. We have not seen any observable difference in specimen characteristics, staining or media performance with longer alcohol exposure times.
How long do I leave the pen residue to dry on the slides before proceeding with the rest of the staining procedure?
The pen residue does contain a solvent that dries within seconds of being placed onto the slide. There is no requirement to wait for the residue to dry. In essence the residue dries the moment it is applied.
How stable is the working solution of the DAB substrate kit (SK-4100) once made up?
The working solution of the DAB substrate kit SK-4100 is stable for up to 6 hours and can be used over this time period without loss of performance. Note that the working solution of our ImmPACT DAB (SK-4105) is 1 week at room temperature and up to 2 weeks if stored in the fridge. The longer stability of the ImmPACT DAB working solution may be beneficial for labs looking to reduce disposal of unused DAB working solutions each day.
I am performing IHC on tissue sections using a mouse IgG primary antibody. My detection system is a biotinylated anti-mouse IgG secondary antibody, a VECTASTAIN® Elite ABC peroxidase kit (PK-6100), in combination with a DAB substrate kit (SK-4100), and I do not see any staining. How do I know if the end detection reagents are working?
Make a fresh working solution of DAB substrate per instructions. Place a small volume (~1 mL) of this DAB substrate into a clean glass test tube. To this 1 ml aliquot, add one drop (~50 µL) of Reagent B only from the VECTASTAIN® Elite ABC kit. If the HRP enzyme is active and the DAB reacts with it, an immediate color change will be observed. This indicates the end detection reagents are working appropriately.
I am using Methyl Green (H-3402) as a counterstain for my IHC, but the stain is a little weak. What suggestions do you have to increase the staining intensity?
Methyl Green counterstain does take a little bit of optimization in some applications. We would suggest heating a volume of the counterstain (~300 ml) to 60 °C and add to a Coplin jar or similar glass staining rack, and submerse the slides. This approach facilitates a better uptake of stain into the section compared with placing the slide on a heated surface and placing drops of stain onto the section. Following the incubation time (~3-5 min), remove slides and wash in tap water. Omit the acetone, acetic acid rinse step described in the instructions and move the slides directly into 95% ethanol for the dehydration, clearing and mounting process. This methodology will retain more stain in the nuclei and hence produce greater intensity.
I have a bottle of the antigen unmasking solution, H-3300. It has been stored in the fridge for a few weeks. When I opened it, it contained some precipitate. Do you know what this precipitate is, and how it may affect product performance?
Product H-3300 is supplied as a highly concentrated (100x) salt solution. It is recommended to be stored in the fridge. In some cases, over time with cold storage, some salts may come out of solution and appear as particulate or precipitated material. Our recommendation is to gently warm the bottle in a warm water bath to re-dissolve the precipitate. Usually 30 min at 35 °C would be sufficient. Once re-dissolved, an aliquot can be drawn from this solution and diluted according to the instructions. Re-dissolving the precipitate maintains the desired pH and salt concentration for optimal performance.
I have Vector Red AP substrate kit, SK-5100. The instructions for use suggest making a working solution with 100-200 mM Tris buffer. Can I use a 50 mM Tris buffer instead?
Vector Red substrate kit (SK-5100) does require a Tris buffer with a minimum of 100 mM to be present for a reaction to satisfactorily occur. It is recommended to use a 200 mM Tris buffer at pH 8.2-8.5 for the most optimal staining results. If a buffer with <100 mM Tris is used to make up the Vector Red working solution it may be that no reaction will occur.
I just received an ImmEdge Pen and when I took the cap off the nib fell out. Is the pen still functional?
Yes. The pen design ensures the nib can move and that the nib is not adhered to the pen itself. The nib needs to move so that once received the nib can be depressed to break the internal membrane to allow residue to flood the nib. In some instances, during shipping, the nib may become dislodged and fall out. Simply put the nib back into the neck of the pen and use as per the supplied instructions. Once the residue floods the nib, the nib material expands slightly to hold the nib within the pen neck.
I recently purchased Normal Goat Serum, Cat. No. S-1000, from Vector Laboratories for use as a general block in my tissue staining applications. What is the percentage of serum, and once diluted, how long can I store the working solution?
Normal Goat Serum, Cat. No. S-1000, is essentially spun whole blood, filtered, heat inactivated and preserved with the addition of sodium azide. The 20 ml provided therefore is all goat serum. For most IHC and IF tissue staining applications, an aliquot is taken and diluted in assay buffer such as PBS or TBS to 2-10% (v/v). This working solution can be stored in the fridge and used for up to one week. Beyond this time, there would be concern for possible microbial contamination that would affect performance.
I see that Vector Laboratories offers three different AEC substrate kits. What is the difference between them, and which should I choose for my tissue staining application?
We have offered conventional AEC peroxidase substrate, Cat. No. SK-4200, for many years. While this is our most economical kit format generating 300 ml of working solution, it is also the least sensitive of the three kits. Use of SK-4200 would be for the detection of abundantly expressed antigens and where assay costs are a main consideration. ImmPACT® AEC, Cat. No. SK-4205, was subsequently introduced and offers several advantages over the conventional SK-4200. In particular, SK-4205 is about 3-5 times greater in sensitivity over SK-4200, and the working solution is stable for two weeks when stored at 4°C. ImmPACT® AMEC Red, Cat. No. SK-4285, is our most sensitive AEC substrate that generates a crisper, brighter red reaction product, and is 5-10 times more sensitive when compared with conventional AEC substrates such as SK-4200. Like ImmPACT® AEC, the ImmPACT® AMEC Red working solution can be stored for two weeks in the fridge without loss of staining performance. We have also determined that where archiving of stained sections is important, ImmPACT® AMEC Red remains localized and undiminished in intensity for at least 2 years when coverslipped with VectaMount® AQ Mounting Medium.
In some IHC detection reagent instructions, the last step states “dehydrate, clear and mount”. What does this mean?
In a manual IHC application, these steps involve submersion of the slides into a graded ethanol series, immediately followed by submersion into a miscible solution (xylene or alternative), followed by placement of a coverslip over the preparation with a suitable mounting medium. These steps preserve the specimen for subsequent visualization, imaging and archiving. From our experience, we suggest moving the slides in a slide rack through a series of dishes, commencing dehydration at 95% ethanol (2 changes for 2 min ea.), then into 100% ethanol (2x 2 min ea.), then into clearing solution (3x 2 min ea.), followed by mounting (coverslipping).
Is there any difference between the horse anti-mouse and anti-rabbit IgG ImmPRESS polymer kits compared to the goat anti-mouse and anti-rabbit IgG ImmPRESS polymer kits?
There is no difference between these kits in terms of performance characteristics such as sensitivity and specificity. We have simply provided the ImmPRESS polymer reagents in different species to accommodate labs with preferences toward using reagents raised in horse or goat.
Is VectaMount Express Mounting Medium compatible with counterstains?
Yes. From our in-house testing, commonly used counterstains such as hematoxylin (e.g. Gill’s and Mayers formulations), nuclear fast red, methyl green and standard H&E (hematoxylin and eosin) stains are compatible with VectaMount Express Mounting Medium.
My enzyme substrate precipitates at the site of localization, but it disappears after the dehydration through alcohol. How can I prevent the enzyme substrate from fading?
We have found that some substrates tend to be a little soluble in lower ethanol grades (i.e. 70% EtOH) during the dehydration step. From our experience, we suggest moving the slides in a slide rack through a series of dishes, commencing dehydration at 95% ethanol (2 changes for 2 min ea.), then into 100% ethanol (2x 2 min ea.), then into clearing solution (3x 2 min ea.), followed by mounting (coverslipping). We recommend diluting 95% ethanol directly from 100% ethanol, instead of using 95% ethanol from a vendor. The ethanol that we use for dehydration is obtained from VWR analytical. The catalog # is BDH1156-4LP (4 L size). It is a mixture of ethanol, methanol (~94-96%) and isopropanol (~4-6%).
Our research lab has an open automated staining platform. Do you know if the ImmPRESS reagents can be applied to an automated system, and if so, do you have any guidelines or procedures?
Yes, we conducted studies on the compatibility of the ImmPRESS HRP polymer reagents with three commercially available autostainers (Agilent/Dako Autostainer Plus, Leica Bond Rx, and Ventana Discovery Ultra). We showed that our reagents are suitable for IHC detection on each of these platforms. The ImmPRESS polymer reagents and enzyme substrates generated equivalent IHC staining results compared to reagents from the instrument manufacturer. Some modifications in the protocol were performed to optimize the signal-to-noise ratio, such as a shorter incubation time for the ImmPRESS polymer and increase in the number of buffer washes following polymer incubation. Please see the following application note for more details about each automated platform:https://vectorlabs.com/media/contentmanager/content/docs/brochures/VL_LIT3023_ImmPRESS_AppNote_4.pdf
Should the VECTASTAIN ABC kit only be stored at 4 degrees C? Can it be stored at -20 degrees C?
We do not recommend freezing the ABC kits. In particular, the ABC reagents exhibit a decrease in enzyme activity and lose some sensitivity when stored at – 20 °C for extended periods.
Since the ImmPRESS polymer reagents are presented as prediluited, ready-to-use solutions, what parameters can I vary to optimize staining conditions?
In most cell and tissue staining applications, little to no modification of the supplied procedure would be required to achieve optimal staining of the target antigen. In circumstances where investigators would like to perform rapid staining or working with wholemounts or thicker (micrometer) specimens, the main parameters to vary would be the incubation time, incubation temperature, and the number and duration of the buffer washing steps between incubations.
What are some considerations when applying a counterstain for immunohistochemistry (IHC)?
The use of a counterstain for IHC is optional and should only be applied if it may be helpful. The idea of using a counterstain is to provide a contrasting color to tissue structures and architecture, compared with the specific target staining generated by the substrate precipitate, to help in the visualization of the overall morphology and cell type in a heterogeneous specimen. As an example, the combination of a brown DAB substrate specific stain with a blue nuclear hematoxylin counterstain is probably the most widely used in standard IHC. A further consideration would be controlling the intensity of the counterstain, usually through incubation time, to ensure it does not obscure the specific substrate stain, thereby generating a false negative result. With regard to this last point, if you are trying to visualize a weakly expressed, possibly transient upregulated target antigen or indeed an antigen of unknown expression in your preparation, omitting a counterstain would be recommended until a baseline (validated positive expression) of specific staining is established. We provide several nuclear counterstain options, and resources such as a counterstain/substrate compatibility table that should help in the selection of an appropriate combination for your application.
What are the advantages of using ImmPRESS® Excel kits compared with one-step peroxidase polymer detection systems?
The ImmPRESS Excel kits are a two-step peroxidase polymer based detection system that include an unconjugated amplifier antibody. This intermediate amplifier antibody increases sensitivity of the assay at least three- to four-fold over that of a one-step polymer based system, by facilitating the introduction of more peroxidase enzyme at the site of specific antigen localization. This increase in sensitivity would be advantageous in instances of weak antigen expression, and to further dilute out an expensive primary antibody. The ImmPRESS Excel Amplifier kits are presented as a complete kit format that include enzyme quench, blocking serum and ImmPACT® DAB EqV peroxidase substrate, in addition to the amplifier antibody and defined ImmPRESS polymer secondary antibody. However, as the two published references below indicate, this format is still modular and allows for the substitution of different detection reagents. These references describe using ImmPACT NovaRED™ peroxidase substrate in combination with the ImmPRESS Excel Amplifier kits. Oncogenesis (2017) 6, e293; doi:10.1038/oncsis.2016.82 Tan, E.M.S., et al (2017) Front. Med. 4:162 (doi: 10.3389/fmed.2017.00162)
What are the components in the antigen unmasking solution, citrate buffer pH 6, H-3300?
The stock solution of H-3300 is supplied as 1M citrate. Once diluted according to the instructions, the working concentration would be 0.01 M citrate. It is essentially a saturated concentrated salt solution.
What are the differences between the regular enzyme substrate kits and the ImmPACT enzyme substrate kits?
The regular substrate kits were first introduced to complement our enzyme-based secondary detection reagents. The regular substrate kits provide an economical approach for staining moderately to highly expressed target antigens. The ImmPACT substrates kits were subsequently introduced with improved sensitivity, more convenient formats and extended stability of the working solution over the regular substrate kits.
What is the capacity of the ImmEdge Hydrophobic Barrier PAP Pen (H-4000)?
The volume of the ImmEdge Pen (H-4000) is about 6 mL. We have not characterized how many times it can be used or overall length of the barrier due to variability in its application.
What is the concentration of DAB chromogen supplied in the DAB substrate kit SK-4100?
The DAB stock solution is provided at 25 mg/ml. Other kit components are Tris buffer (1.25 M), Hydrogen peroxide (1.5%) and NiCl2 (5.0%).
What is the difference between the Avidin/Biotin Blocking Kit, Cat. No. SP-2001, and the Streptavidin/Biotin Blocking Kit, Cat. No. SP-2002, and can they be used interchangeably?
These kits were developed to prevent non-specific binding of avidin or streptavidin based detection reagents primarily to endogenous biotin in cell and tissue IHC and IF applications. It is generally recommended to match the blocking and detection reagent such as using an avidin/biotin blocking kit with an avidin based detection system. In most cases, however, these kits can be used interchangeably, except in circumstances where avidin or streptavidin may bind to inherent non-biotin associated structures (see: Alon, R., et al {1992}, Eur. J. Cell Biol. 58:271-279).
What is the difference between the R.T.U. Animal Free Block and Diluent (SP-5035) and the 5x Animal Free Blocker® (SP-5030)?
Both products are derived from the same plant protein and in some applications can be used interchangeably. At a high level, one is presented as a ready-to-use (RTU) format and the other a more economical concentrate. The key difference is that the RTU format has been specifically optimized for use in IHC and IF applications. The RTU format has a neutral pH suitable for tissue and cell-based assays and has been more highly refined to avoid the presence of flocculent material that may cause interference with microscopy work. The 5x concentrated format is best suited for membrane blotting applications.
What is the difference between the VECTASTAIN ABC regular peroxidase kits (PK-4000 series) and the VECTASTAIN Elite ABC peroxidase kits (PK-6000 series)?Can the reagents from a regular ABC kit be interchanged with reagents from an Elite ABC kit?
The key difference between the VECTASTAIN ABC regular kits and the VECTASTAIN Elite ABC kits is sensitivity. The VECTASTAIN Elite ABC kits are 5x greater in sensitivity than the regular kits. This increased sensitivity allows for further dilution of a potentially expensive primary antibody and detection of weaker (lower abundance) expressed target antigen. This difference in sensitivity is achieved through slightly different chemistry in the VECTASTAIN Elite ABC reagents that introduces more peroxidase enzyme and hence generates a greater reaction with the substrate and color deposition at the site of antigen localization. The sera and secondary antibodies in the VECTASTAIN regular and Elite ABC kits are the same and can be used interchangeably. However Reagent A (avidin) and Reagent B (biotinylated enzyme) are specific for the kit and are not interchangeable.
What is the stability of the working solutions of the VECTASTAIN ABC kits once made up according to the instructions?
We recommend using the working solution of the diluted ABC reagent (mixing Reagent A and Reagent B together) within 24 hour of being made to retain maximum enzyme activity and performance. This will allow for comparison of staining results between assays. In the species-specific kits, diluted (working solution) of the sera and biotinylated secondary antibodies can be kept in the fridge for up to 1 week. Note that Ready-To-Use (RTU) formats of the VECTASTAIN ABC reagents are offered for greater convenience and stability of the working solutions.
What is the width of the nib/tip supplied with the ImmEdge pens?
The nib provided with each ImmEdge Pen is 3 mm wide.
Why am I getting air bubbles under the coverslip after mounting with Vectamount Express?
Usually the presence of air bubbles can be greatly reduced, and even eliminated, when using a manual approach, by carefully lowering the coverslip slowly over the specimen. Sometimes this may take a little practice to perfect.
Advice? I’m new to immunohistochemistry, do you have any recommendations for how best to get started?
Yes we do. Please visit our comprehensive Immunohistochemistry (IHC) page for assistance with each step of the IHC workflow. This resource contains a great deal of technical material including product recommendations and links to other technical documentation / videos / webinars.
Could you please describe what would be an appropriate negative control for IHC that can be used to help validate tissue staining results?
A commonly used negative control is omission of the primary antibody. While this control addresses whether the secondary antibody reagents are a source of staining, inadvertent binding of the primary antibody to the tissue can occur. Various tissue elements such as Fc receptors and charged molecules may bind the primary antibody non-specifically. Simply omitting the primary antibody as a negative control would miss potential false positive staining by this means. A few “publication worthy” negative controls for IHC are listed below: 1) Preabsorption of primary antibody with the immunogen used to generate the antibody can be employed. The working dilution of the primary antibody and an optimized concentration of the immunogen are incubated together for a period prior to application to the specimen. Lack of staining would indicate specificity of the primary antibody to the target antigen in solution. Positive staining using this method, however, may indicate lack of specificity of the primary antibody and/or the primary antibody is being bound by tissue elements. To rule out the latter, this control can be used in combination with suggestion no. 2 below. Note that adsorption controls are not always feasible or practical depending on the cost or source of the immunogen. 2) Use of an isotype control (e.g. “non-immune” mouse IgG), matched to that of the primary antibody and applied at the same protein concentration as the primary antibody, is probably the most widely used negative control. This control addresses whether tissue elements are inadvertently binding immunoglobulin from the same species as the primary antibody, in addition to non-specific binding from the secondary detection reagents. In most cases, use of a sub-class of isotype immunoglobulin (e.g. mouse IgG2a or IgG2b) is not required. Note that the use of pre-immune immunoglobulin, obtained prior to immunization, could also be used. However, it is very unusual for commercial vendors to offer pre-immune immunoglobulin. 3) Substitution of the primary antibody with an “irrelevant antibody” is also a suitable negative control. The term “irrelevant” refers to a primary antibody of the same isotype as the specific primary antibody (i.e. mouse IgG) and applied at the same concentration, that is known not to bind to a target in the tissue specimen. An example would be an anti-cytokeratin antibody on smooth muscle tissue. As with negative control no. 1 above, lack of staining indicates tissue elements are not binding this isotype of immunoglobulin. 4) In some cases, the target antigen can be removed from the tissue specimen as a sort of “knock-out” preparation. Once the target antigen has been removed, the complete assay is run to determine lack of staining. One method to remove the target antigen is using defined enzyme digestion. Examples include the use of a collagenase if the target antigen is collagen, or hyaluronidase if the target antigen is hyaluronic acid. Of course, there are limitations to this approach, however, variations of this “deletion” or “knock-out” approach would be valid negative controls.
For my IHC application, I intend to apply a mouse IgG primary antibody on rat tissue sections. Should I use the Vector Mouse on Mouse detection kit?
Vector Laboratories’ Mouse on Mouse detection kits (M.O.M.®) are designed for the detection of mouse IgG primary antibodies on mouse tissue sections. For detecting mouse primary antibodies on rat tissue, we offer a selection of anti-mouse, rat adsorbed secondary detection reagents. These are intended to be used for this application and would generate the most optimal signal to noise staining ratio. We offer a biotinylated anti-mouse IgG, rat adsorbed secondary antibody (Cat. No. BA-2001) for use with VECTASTAIN® ABC kits or avidin and streptavidin enzyme conjugates. Alternatively, we offer an ImmPRESS® HRP polymer anti-mouse IgG, rat adsorbed detection kit (Cat. No. MP-7422) for a convenient, one-step IHC methodology.
How do I use PBS or TBS buffer to remount my slide using VectaMount AQ Aqueous Mounting Medium?
We would suggest using a Coplin jar or slide rack to completely submerge the slides/coverslipped sections in PBS or TBS buffer. Place the slides on their side or on their end and not lying flat. We would suggest leaving them submerged for an extended period, which may be overnight or longer (48-72 hours).
I am performing IHC on tissue sections using a mouse IgG primary antibody. My detection system is a biotinylated anti-mouse IgG secondary antibody, a VECTASTAIN® Elite ABC peroxidase kit (PK-6100), in combination with a DAB substrate kit (SK-4100), and I do not see any staining. How do I know if the end detection reagents are working?
Make a fresh working solution of DAB substrate per instructions. Place a small volume (~1 mL) of this DAB substrate into a clean glass test tube. To this 1 ml aliquot, add one drop (~50 µL) of Reagent B only from the VECTASTAIN® Elite ABC kit. If the HRP enzyme is active and the DAB reacts with it, an immediate color change will be observed. This indicates the end detection reagents are working appropriately.
I am using Methyl Green (H-3402) as a counterstain for my IHC, but the stain is a little weak. What suggestions do you have to increase the staining intensity?
Methyl Green counterstain does take a little bit of optimization in some applications. We would suggest heating a volume of the counterstain (~300 ml) to 60 °C and add to a Coplin jar or similar glass staining rack, and submerse the slides. This approach facilitates a better uptake of stain into the section compared with placing the slide on a heated surface and placing drops of stain onto the section. Following the incubation time (~3-5 min), remove slides and wash in tap water. Omit the acetone, acetic acid rinse step described in the instructions and move the slides directly into 95% ethanol for the dehydration, clearing and mounting process. This methodology will retain more stain in the nuclei and hence produce greater intensity.
I have a bottle of the antigen unmasking solution, H-3300. It has been stored in the fridge for a few weeks. When I opened it, it contained some precipitate. Do you know what this precipitate is, and how it may affect product performance?
Product H-3300 is supplied as a highly concentrated (100x) salt solution. It is recommended to be stored in the fridge. In some cases, over time with cold storage, some salts may come out of solution and appear as particulate or precipitated material. Our recommendation is to gently warm the bottle in a warm water bath to re-dissolve the precipitate. Usually 30 min at 35 °C would be sufficient. Once re-dissolved, an aliquot can be drawn from this solution and diluted according to the instructions. Re-dissolving the precipitate maintains the desired pH and salt concentration for optimal performance.
In some IHC detection reagent instructions, the last step states “dehydrate, clear and mount”. What does this mean?
In a manual IHC application, these steps involve submersion of the slides into a graded ethanol series, immediately followed by submersion into a miscible solution (xylene or alternative), followed by placement of a coverslip over the preparation with a suitable mounting medium. These steps preserve the specimen for subsequent visualization, imaging and archiving. From our experience, we suggest moving the slides in a slide rack through a series of dishes, commencing dehydration at 95% ethanol (2 changes for 2 min ea.), then into 100% ethanol (2x 2 min ea.), then into clearing solution (3x 2 min ea.), followed by mounting (coverslipping).
My enzyme substrate precipitates at the site of localization, but it disappears after the dehydration through alcohol. How can I prevent the enzyme substrate from fading?
We have found that some substrates tend to be a little soluble in lower ethanol grades (i.e. 70% EtOH) during the dehydration step. From our experience, we suggest moving the slides in a slide rack through a series of dishes, commencing dehydration at 95% ethanol (2 changes for 2 min ea.), then into 100% ethanol (2x 2 min ea.), then into clearing solution (3x 2 min ea.), followed by mounting (coverslipping). We recommend diluting 95% ethanol directly from 100% ethanol, instead of using 95% ethanol from a vendor. The ethanol that we use for dehydration is obtained from VWR analytical. The catalog # is BDH1156-4LP (4 L size). It is a mixture of ethanol, methanol (~94-96%) and isopropanol (~4-6%).
What are some considerations when applying a counterstain for immunohistochemistry (IHC)?
The use of a counterstain for IHC is optional and should only be applied if it may be helpful. The idea of using a counterstain is to provide a contrasting color to tissue structures and architecture, compared with the specific target staining generated by the substrate precipitate, to help in the visualization of the overall morphology and cell type in a heterogeneous specimen. As an example, the combination of a brown DAB substrate specific stain with a blue nuclear hematoxylin counterstain is probably the most widely used in standard IHC. A further consideration would be controlling the intensity of the counterstain, usually through incubation time, to ensure it does not obscure the specific substrate stain, thereby generating a false negative result. With regard to this last point, if you are trying to visualize a weakly expressed, possibly transient upregulated target antigen or indeed an antigen of unknown expression in your preparation, omitting a counterstain would be recommended until a baseline (validated positive expression) of specific staining is established. We provide several nuclear counterstain options, and resources such as a counterstain/substrate compatibility table that should help in the selection of an appropriate combination for your application.
What are the components in the antigen unmasking solution, citrate buffer pH 6, H-3300?
The stock solution of H-3300 is supplied as 1M citrate. Once diluted according to the instructions, the working concentration would be 0.01 M citrate. It is essentially a saturated concentrated salt solution.
What is the capacity of the ImmEdge Hydrophobic Barrier PAP Pen (H-4000)?
The volume of the ImmEdge Pen (H-4000) is about 6 mL. We have not characterized how many times it can be used or overall length of the barrier due to variability in its application.
After mounting with VectaMount Express, why do my slides look cloudy?
It is important to use 99+% alcohol (isopropanol or ethanol) for the brief dehydration steps. Lower alcohol grades will not be viable substitutes. Slides should be rinsed in tap water (not buffer) prior to dehydration, as salts in buffers can precipitate if not removed. Depending on slide volume, alcohol baths should be changed out on a regular basis with fresh solution. It is suggested to drain excess water when transferring slides from tap water into the alcohol bath.
Can I dry the Vectamount Express mounted slides in an oven?
No. It is recommended to dry slides at ambient room temperature.
How can I successfully remove the coverslip once it has been applied to a slide with VectaMount Express Mounting Medium?
Place the slides in a glass staining rack or Coplin jar and completely submerge the slides in xylene overnight. After this time the coverslip should be easy to remove from the slide.
How long can the slides remain in isopropanol before mounting with Vectamount Express?
There are no specific time limits for slides to be in the alcohol bath. Depending on the number of slides in a given assay, the last slide to be mounted will be in the alcohol bath longer than the first mounted slide. This may take several minutes or much longer. We have not seen any observable difference in specimen characteristics, staining or media performance with longer alcohol exposure times.
Is VectaMount Express Mounting Medium compatible with counterstains?
Yes. From our in-house testing, commonly used counterstains such as hematoxylin (e.g. Gill’s and Mayers formulations), nuclear fast red, methyl green and standard H&E (hematoxylin and eosin) stains are compatible with VectaMount Express Mounting Medium.
Why am I getting air bubbles under the coverslip after mounting with Vectamount Express?
Usually the presence of air bubbles can be greatly reduced, and even eliminated, when using a manual approach, by carefully lowering the coverslip slowly over the specimen. Sometimes this may take a little practice to perfect.
Can I perform double staining for two different antigens on the same section using two mouse primary antibodies with the same VECTASTAIN ABC Mouse IgG Kit?
Yes. We do offer complete protocols, helpful tips as well as enzyme substrate combination suggestions and images on our website for this application at the following link: https://go.vectorlabs.com/multiplexing. Essentially the staining is performed sequentially (one stain after the other) from primary antibody right through to substrate color development using two different enzyme substrates.
How do I choose which VECTASTAIN ABC Kit to use?
We offer a choice of either peroxidase or alkaline phosphatase based VECTASTAIN ABC kit detection systems. Selection of which enzyme system to use would be the first step. Most IHC applications involve detecting an unconjugated primary antibody. Consider the species in which the primary antibody is raised (e.g. rabbit), and then match to to corresponding species-specific VECTASTAIN ABC kit (e.g. VECTASTAIN ABC Rabbit IgG kit). If you already have a biotinylated target (i.e. biotinylated secondary antibody or primary antibody), then a standard VECTASTAIN ABC kit containing just the avidin/biotinylated enzyme reagents would be required. Note that only the VECTASTAIN Universal Elite PLUS ABC kit (PK-8200) contains a substrate.
What is the difference between the VECTASTAIN ABC regular peroxidase kits (PK-4000 series) and the VECTASTAIN Elite ABC peroxidase kits (PK-6000 series)?Can the reagents from a regular ABC kit be interchanged with reagents from an Elite ABC kit?
The key difference between the VECTASTAIN ABC regular kits and the VECTASTAIN Elite ABC kits is sensitivity. The VECTASTAIN Elite ABC kits are 5x greater in sensitivity than the regular kits. This increased sensitivity allows for further dilution of a potentially expensive primary antibody and detection of weaker (lower abundance) expressed target antigen. This difference in sensitivity is achieved through slightly different chemistry in the VECTASTAIN Elite ABC reagents that introduces more peroxidase enzyme and hence generates a greater reaction with the substrate and color deposition at the site of antigen localization. The sera and secondary antibodies in the VECTASTAIN regular and Elite ABC kits are the same and can be used interchangeably. However Reagent A (avidin) and Reagent B (biotinylated enzyme) are specific for the kit and are not interchangeable.
What is the stability of the working solutions of the VECTASTAIN ABC kits once made up according to the instructions?
We recommend using the working solution of the diluted ABC reagent (mixing Reagent A and Reagent B together) within 24 hour of being made to retain maximum enzyme activity and performance. This will allow for comparison of staining results between assays. In the species-specific kits, diluted (working solution) of the sera and biotinylated secondary antibodies can be kept in the fridge for up to 1 week. Note that Ready-To-Use (RTU) formats of the VECTASTAIN ABC reagents are offered for greater convenience and stability of the working solutions.
Can I use the same ImmPRESS anti-mouse IgG polymer reagent (MP-7402) to perform double labeling with two different mouse primary antibodies on the same tissue section?
Yes, the same ImmPRESS peroxidase secondary detection reagent can be used for double staining. There are a few considerations. First, we would suggest optimizing each single stain first on different tissue sections. Once the conditions for each stain have been established, the single assays should be run sequentially, from primary antibody though substrate color development, to achieve double staining on the same section. Secondly, different peroxidase substrates will have to be used to provide adequate color contrast and reliable target antigen localization. This double staining approach is most reproducible when detecting antigens expressed in different cell types on the same section, or different cell compartments of the same cell type. Please see our multiple antigen labeling guide for additional considerations:
Is there any difference between the horse anti-mouse and anti-rabbit IgG ImmPRESS polymer kits compared to the goat anti-mouse and anti-rabbit IgG ImmPRESS polymer kits?
There is no difference between these kits in terms of performance characteristics such as sensitivity and specificity. We have simply provided the ImmPRESS polymer reagents in different species to accommodate labs with preferences toward using reagents raised in horse or goat.
Our research lab has an open automated staining platform. Do you know if the ImmPRESS reagents can be applied to an automated system, and if so, do you have any guidelines or procedures?
Yes, we conducted studies on the compatibility of the ImmPRESS HRP polymer reagents with three commercially available autostainers (Agilent/Dako Autostainer Plus, Leica Bond Rx, and Ventana Discovery Ultra). We showed that our reagents are suitable for IHC detection on each of these platforms. The ImmPRESS polymer reagents and enzyme substrates generated equivalent IHC staining results compared to reagents from the instrument manufacturer. Some modifications in the protocol were performed to optimize the signal-to-noise ratio, such as a shorter incubation time for the ImmPRESS polymer and increase in the number of buffer washes following polymer incubation. Please see the following application note for more details about each automated platform:https://vectorlabs.com/media/contentmanager/content/docs/brochures/VL_LIT3023_ImmPRESS_AppNote_4.pdf
Since the ImmPRESS polymer reagents are presented as prediluited, ready-to-use solutions, what parameters can I vary to optimize staining conditions?
In most cell and tissue staining applications, little to no modification of the supplied procedure would be required to achieve optimal staining of the target antigen. In circumstances where investigators would like to perform rapid staining or working with wholemounts or thicker (micrometer) specimens, the main parameters to vary would be the incubation time, incubation temperature, and the number and duration of the buffer washing steps between incubations.
What are the advantages of using ImmPRESS® Excel kits compared with one-step peroxidase polymer detection systems?
The ImmPRESS Excel kits are a two-step peroxidase polymer based detection system that include an unconjugated amplifier antibody. This intermediate amplifier antibody increases sensitivity of the assay at least three- to four-fold over that of a one-step polymer based system, by facilitating the introduction of more peroxidase enzyme at the site of specific antigen localization. This increase in sensitivity would be advantageous in instances of weak antigen expression, and to further dilute out an expensive primary antibody. The ImmPRESS Excel Amplifier kits are presented as a complete kit format that include enzyme quench, blocking serum and ImmPACT® DAB EqV peroxidase substrate, in addition to the amplifier antibody and defined ImmPRESS polymer secondary antibody. However, as the two published references below indicate, this format is still modular and allows for the substitution of different detection reagents. These references describe using ImmPACT NovaRED™ peroxidase substrate in combination with the ImmPRESS Excel Amplifier kits. Oncogenesis (2017) 6, e293; doi:10.1038/oncsis.2016.82 Tan, E.M.S., et al (2017) Front. Med. 4:162 (doi: 10.3389/fmed.2017.00162)
How stable is the working solution of the DAB substrate kit (SK-4100) once made up?
The working solution of the DAB substrate kit SK-4100 is stable for up to 6 hours and can be used over this time period without loss of performance. Note that the working solution of our ImmPACT DAB (SK-4105) is 1 week at room temperature and up to 2 weeks if stored in the fridge. The longer stability of the ImmPACT DAB working solution may be beneficial for labs looking to reduce disposal of unused DAB working solutions each day.
I have Vector Red AP substrate kit, SK-5100. The instructions for use suggest making a working solution with 100-200 mM Tris buffer. Can I use a 50 mM Tris buffer instead?
Vector Red substrate kit (SK-5100) does require a Tris buffer with a minimum of 100 mM to be present for a reaction to satisfactorily occur. It is recommended to use a 200 mM Tris buffer at pH 8.2-8.5 for the most optimal staining results. If a buffer with <100 mM Tris is used to make up the Vector Red working solution it may be that no reaction will occur.
I see that Vector Laboratories offers three different AEC substrate kits. What is the difference between them, and which should I choose for my tissue staining application?
We have offered conventional AEC peroxidase substrate, Cat. No. SK-4200, for many years. While this is our most economical kit format generating 300 ml of working solution, it is also the least sensitive of the three kits. Use of SK-4200 would be for the detection of abundantly expressed antigens and where assay costs are a main consideration. ImmPACT® AEC, Cat. No. SK-4205, was subsequently introduced and offers several advantages over the conventional SK-4200. In particular, SK-4205 is about 3-5 times greater in sensitivity over SK-4200, and the working solution is stable for two weeks when stored at 4°C. ImmPACT® AMEC Red, Cat. No. SK-4285, is our most sensitive AEC substrate that generates a crisper, brighter red reaction product, and is 5-10 times more sensitive when compared with conventional AEC substrates such as SK-4200. Like ImmPACT® AEC, the ImmPACT® AMEC Red working solution can be stored for two weeks in the fridge without loss of staining performance. We have also determined that where archiving of stained sections is important, ImmPACT® AMEC Red remains localized and undiminished in intensity for at least 2 years when coverslipped with VectaMount® AQ Mounting Medium.
What are the differences between the regular enzyme substrate kits and the ImmPACT enzyme substrate kits?
The regular substrate kits were first introduced to complement our enzyme-based secondary detection reagents. The regular substrate kits provide an economical approach for staining moderately to highly expressed target antigens. The ImmPACT substrates kits were subsequently introduced with improved sensitivity, more convenient formats and extended stability of the working solution over the regular substrate kits.
What is the concentration of DAB chromogen supplied in the DAB substrate kit SK-4100?
The DAB stock solution is provided at 25 mg/ml. Other kit components are Tris buffer (1.25 M), Hydrogen peroxide (1.5%) and NiCl2 (5.0%).
Do I have to apply the avidin/biotin blocking kit reagents before the primary antibody?
If the primary antibody is unconjugated, then the avidin/biotin blocking kit reagents can be applied at any time prior to the application of the biotinylated secondary antibody. If the primary antibody is biotinylated, then we would recommend applying the avidin/biotin blocking kit reagents before primary antibody incubation.
Does BLOXALL quench intestinal alkaline phosphatase activity?
Yes. BLOXALL quenches endogenous peroxidase enzyme activity in addition to all alkaline phosphatase (AP) isoforms including intestinal AP.
I recently purchased Normal Goat Serum, Cat. No. S-1000, from Vector Laboratories for use as a general block in my tissue staining applications. What is the percentage of serum, and once diluted, how long can I store the working solution?
Normal Goat Serum, Cat. No. S-1000, is essentially spun whole blood, filtered, heat inactivated and preserved with the addition of sodium azide. The 20 ml provided therefore is all goat serum. For most IHC and IF tissue staining applications, an aliquot is taken and diluted in assay buffer such as PBS or TBS to 2-10% (v/v). This working solution can be stored in the fridge and used for up to one week. Beyond this time, there would be concern for possible microbial contamination that would affect performance.
What is the difference between the Avidin/Biotin Blocking Kit, Cat. No. SP-2001, and the Streptavidin/Biotin Blocking Kit, Cat. No. SP-2002, and can they be used interchangeably?
These kits were developed to prevent non-specific binding of avidin or streptavidin based detection reagents primarily to endogenous biotin in cell and tissue IHC and IF applications. It is generally recommended to match the blocking and detection reagent such as using an avidin/biotin blocking kit with an avidin based detection system. In most cases, however, these kits can be used interchangeably, except in circumstances where avidin or streptavidin may bind to inherent non-biotin associated structures (see: Alon, R., et al {1992}, Eur. J. Cell Biol. 58:271-279).
What is the difference between the R.T.U. Animal Free Block and Diluent (SP-5035) and the 5x Animal Free Blocker® (SP-5030)?
Both products are derived from the same plant protein and in some applications can be used interchangeably. At a high level, one is presented as a ready-to-use (RTU) format and the other a more economical concentrate. The key difference is that the RTU format has been specifically optimized for use in IHC and IF applications. The RTU format has a neutral pH suitable for tissue and cell-based assays and has been more highly refined to avoid the presence of flocculent material that may cause interference with microscopy work. The 5x concentrated format is best suited for membrane blotting applications.
Can I apply the ImmEdge Pen residue to wet slides?
Yes. The ImmEdge Pen residue contains a wax constituent that allows for application to microscope slides that have buffer or similar aqueous solutions on the surface. Applying the pen residue to wet slides does not affect adhesion of the residue nor interfere with the tissue section.
Does the ImmEdge Pen residue fluoresce?
Yes, the pen residue does fluoresce. However, this property does not limit the pens use to only light microscope applications. We routinely use the ImmEdge pen for immunofluorescent tissue staining applications. Usually the pen residue is applied well outside of the tissue section perimeter, and as such, the inherent fluorescent properties of the pen residue do not interfere with specific fluorescent signal.
How long do I leave the pen residue to dry on the slides before proceeding with the rest of the staining procedure?
The pen residue does contain a solvent that dries within seconds of being placed onto the slide. There is no requirement to wait for the residue to dry. In essence the residue dries the moment it is applied.
I just received an ImmEdge Pen and when I took the cap off the nib fell out. Is the pen still functional?
Yes. The pen design ensures the nib can move and that the nib is not adhered to the pen itself. The nib needs to move so that once received the nib can be depressed to break the internal membrane to allow residue to flood the nib. In some instances, during shipping, the nib may become dislodged and fall out. Simply put the nib back into the neck of the pen and use as per the supplied instructions. Once the residue floods the nib, the nib material expands slightly to hold the nib within the pen neck.
What is the width of the nib/tip supplied with the ImmEdge pens?
The nib provided with each ImmEdge Pen is 3 mm wide.
- Immunofluorescence
- VECTASHIELD Antifade Mounting Media
- TrueVIEW Autofluorescence Quenching Kit
How quickly can I view sections after mounting with VECTASHIELD® Vibrance (catalog # H-1700)? Is it recommended to wait one hour?
Sections can be viewed immediately after mounting with VECTASHIELD® Vibrance, but one hour is recommended for optimal signal intensity.
Can VECTASHIELD® be used for super resolution microscopy?
Of the many variations of super resolution microscopy (SRM) currently being used, investigators have found the properties of product H-1000 (VECTASHIELD), to be advantageous in stochastic optical reconstruction microscopy (STORM) and structured illumination microscopy (SIM). Refer to the following published references for further information: 1) Olivier, N., et al (2013) Simple buffers for 3D STORM microscopy. Biochemical Optics Express 4:885-899. 2) Wegel., et al (2016) Imaging cellular structuires in super-resolution with SIM, STED and localisation microscopy: A practical comparison. Scientific Reports 6:27290.
Do I need to dehydrate the tissue sections before applying VECTASHIELD?
No form of tissue dehydration (e.g. air drying or ethanol exposure) is required nor recommended when applying VECTASHIELD. From our experience, the most optimal antifade actions of VECTASHIELD are obtained when the preparation is removed from the final buffer/water rinse, kept slightly wet/moist and then coverslipped with a small volume (25-50 uL) of VECTASHIELD.
Does the coverslip need to be sealed with nail polish or similar compound after mounting?
Similar to other non-setting media, we recommend you to seal the coverslip perimeter if the intention is to store the slides for a period of time.
Does VECTASHIELD contain glycerol?
All of the VECTASHIELD formats we currently offer which includes regular non-setting VECTASHIELD, PLUS, HardSet and Vibrance formulations each contain a percentage of glycerol.
For the wash step after applying the TrueVIEW quenching reagent, can I use buffers other than PBS or detergents?
No, we have found that the TrueVIEW reagent lifts off the tissue using TBS or HEPES buffer. Detergents are incompatible.
How long can I store the TrueVIEW working solution?
Once made up, the working solution of TrueVIEW can be stored either on the bench top or in the fridge (2-8C) fro about 48 hours (2 days) withut loss of activity or function. Following this time we would suggest discarding unused working solution and make fresh solution as required.
How long does it take for the VECTASHIELD® Vibrance to cure?
With adequate removal of excess buffer, the VECTASHIELD Vibrance will cure enough by one hour to hold the coverslip in place. Complete curing takes about 4 – 24 hours at room temperature.
I am using VECTASHIELD® HardSet™ Mounting Medium with DAPI (Cat No. H-1500). The sections look good initially, however I do see bubble or vacuole formation occur under the coverslip after some time. Do you have any suggestions on how this situation can be remedied?
Fixed, thin cut (<10 um), glass mounted sections are recommended to be used in combination with this medium. Unfixed material, thicker sections or the use of gasket or chamber slides or well formats may increase the incidence of bubble formation when applying H-1500. If using thin sections as described, ensure an adequate volume of the medium is being applied to spread out under the coverslip. Storage of slides in the freezer (-20 °C) may also reduce the incidence of bubbles. For existing mounted sections with bubble formation, coverslips can be removed by soaking the slides in buffer, and then remounted using fresh media.
Is TrueVIEW effective against lipofuscin derived autofluorescence?
The working solution of TrueVIEW works in an electrostatic manner to greatly reduce or eliminate fluorescence in tissue sections induced through the use of an aldehyde based fixative. TrueVIEW is also effective at reducing fluorescence from tissue components such as collagen, elastin and red blood cells. TureVIEW does not work to reduce autofluorescence due to lipofuscin.
Do I need to seal the perimeter of the coverslip after I apply VECTASHIELD?
That depends upon how long you wish to retain the slides and which VECTASHIELD formulation you are using. If you are using one of our non-setting VECTASHIELD products such as H-1000/H-1200 or H-1900/H-2000, then we suggest sealing the coverslip with plastic sealant or nail polish if you intend to keep the slides beyond a day or so. If you are using one of our setting/curing formulations such as VECTASHIELD HardSet or Vibrance, then in most cases when using thin cut (<10 um) tissue sections or cell monolayers, no sealing of the coverslip is requuired.
Is VECTASHIELD PLUS suitable for super resolution microscopy (SRM)?
We do know that regular VECTASHIELD antifade mounting media (H-1000 & H-1200) are suitable for SRM. However, the formulation for VECTASHIELD PLUS is different from that used for regular VECTASHIELD. Indeed one component of H-1000 and H-1200 that may have been a contributing factor to the underlying blue tone and that appears to play a key role in optimal signal for SRM, has been removed from VECTASHIELD PLUS. Therefore, until we have data to the contrary VECTASHIELD PLUS would not be recommended for SRM.
My tissue sections turned blue when I applied TrueVIEW. Is this supposed to happen?
Yes, the working solution is a blue color that does “stain” the tissue section blue. This indicates an active and appropriate chemical reaction is occurring. The blue stain on the section does not fluoresce and does not interfere with the immunofluorescence application.
What are the advantages of VECTASHIELD PLUS over regular VECTASHIELD (H-1000 & H-1200)?
VECTASHIELD PLUS provides several advantages over the regular VECTASHIELD (H-1000 & H-1200). In particular, VECTASHIELD PLUS media have significantly lower inherent background toning and provide superior signal retention particularly in the far-red spectrum (e.g. Cy5).
What mounting media can I use with TrueVIEW?
Both TrueVIEW products, SP-8400 and SP-8500, are supplied with 2 mL of VECTASHIELD Vibrance antifade mounting media. We have found that the mounting media does play a crucial role in maintaining the high signal to noise ratio when using TrueVIEW. VECTASHIELD Vibrance is included therefore as a critical component of the kit and it is recommended to use the media supplied for optimal results. At this time we do not have sufficient data to confidently recommend the use of other vendors mounting media with TrueVIEW. Substitution of VECTASHIELD Vibrance with another mounting media may result in less than satisfactory results.
Why is the TrueVIEW™ Autofluorescence Quenching reagent applied after completion of the IF assay and not at the start of the procedure?
During product development, applying the TrueVIEW™ Quenching reagent at the end of our standard IF procedure yielded the most optimal reduction in autofluorescence signal. The reagent is retained on the tissue at the time of mounting, allowing for extended quenching action, with little to no effect on the specific fluorescent signal. However, in applications with very brief staining procedures, such as a primary antibody directly conjugated with a fluorophore, application of TrueVIEW™ Quenching reagent may be just as effective at the start of the procedure.
How quickly can I view sections after mounting with VECTASHIELD® Vibrance (catalog # H-1700)? Is it recommended to wait one hour?
Sections can be viewed immediately after mounting with VECTASHIELD® Vibrance, but one hour is recommended for optimal signal intensity.
Can VECTASHIELD® be used for super resolution microscopy?
Of the many variations of super resolution microscopy (SRM) currently being used, investigators have found the properties of product H-1000 (VECTASHIELD), to be advantageous in stochastic optical reconstruction microscopy (STORM) and structured illumination microscopy (SIM). Refer to the following published references for further information: 1) Olivier, N., et al (2013) Simple buffers for 3D STORM microscopy. Biochemical Optics Express 4:885-899. 2) Wegel., et al (2016) Imaging cellular structuires in super-resolution with SIM, STED and localisation microscopy: A practical comparison. Scientific Reports 6:27290.
Do I need to dehydrate the tissue sections before applying VECTASHIELD?
No form of tissue dehydration (e.g. air drying or ethanol exposure) is required nor recommended when applying VECTASHIELD. From our experience, the most optimal antifade actions of VECTASHIELD are obtained when the preparation is removed from the final buffer/water rinse, kept slightly wet/moist and then coverslipped with a small volume (25-50 uL) of VECTASHIELD.
Do I need to seal the perimeter of the coverslip after I apply VECTASHIELD?
That depends upon how long you wish to retain the slides and which VECTASHIELD formulation you are using. If you are using one of our non-setting VECTASHIELD products such as H-1000/H-1200 or H-1900/H-2000, then we suggest sealing the coverslip with plastic sealant or nail polish if you intend to keep the slides beyond a day or so. If you are using one of our setting/curing formulations such as VECTASHIELD HardSet or Vibrance, then in most cases when using thin cut (<10 um) tissue sections or cell monolayers, no sealing of the coverslip is requuired.
Does the coverslip need to be sealed with nail polish or similar compound after mounting?
Similar to other non-setting media, we recommend you to seal the coverslip perimeter if the intention is to store the slides for a period of time.
Does VECTASHIELD contain glycerol?
All of the VECTASHIELD formats we currently offer which includes regular non-setting VECTASHIELD, PLUS, HardSet and Vibrance formulations each contain a percentage of glycerol.
How long does it take for the VECTASHIELD® Vibrance to cure?
With adequate removal of excess buffer, the VECTASHIELD Vibrance will cure enough by one hour to hold the coverslip in place. Complete curing takes about 4 – 24 hours at room temperature.
I am using VECTASHIELD® HardSet™ Mounting Medium with DAPI (Cat No. H-1500). The sections look good initially, however I do see bubble or vacuole formation occur under the coverslip after some time. Do you have any suggestions on how this situation can be remedied?
Fixed, thin cut (<10 um), glass mounted sections are recommended to be used in combination with this medium. Unfixed material, thicker sections or the use of gasket or chamber slides or well formats may increase the incidence of bubble formation when applying H-1500. If using thin sections as described, ensure an adequate volume of the medium is being applied to spread out under the coverslip. Storage of slides in the freezer (-20 °C) may also reduce the incidence of bubbles. For existing mounted sections with bubble formation, coverslips can be removed by soaking the slides in buffer, and then remounted using fresh media.
Is VECTASHIELD PLUS suitable for super resolution microscopy (SRM)?
We do know that regular VECTASHIELD antifade mounting media (H-1000 & H-1200) are suitable for SRM. However, the formulation for VECTASHIELD PLUS is different from that used for regular VECTASHIELD. Indeed one component of H-1000 and H-1200 that may have been a contributing factor to the underlying blue tone and that appears to play a key role in optimal signal for SRM, has been removed from VECTASHIELD PLUS. Therefore, until we have data to the contrary VECTASHIELD PLUS would not be recommended for SRM.
What are the advantages of VECTASHIELD PLUS over regular VECTASHIELD (H-1000 & H-1200)?
VECTASHIELD PLUS provides several advantages over the regular VECTASHIELD (H-1000 & H-1200). In particular, VECTASHIELD PLUS media have significantly lower inherent background toning and provide superior signal retention particularly in the far-red spectrum (e.g. Cy5).
Do you have any published references describing the use of TrueVIEW Autofluorescence Quenching Kit?
Yes, there are a number of published references describing the use of TrueVIEW Autofluorescence Quenching Kit in the scientific literature. Please refer to a partial list of these publications in the Technical Information section of the product detail page for SP-8400.
For the wash step after applying the TrueVIEW quenching reagent, can I use buffers other than PBS or detergents?
No, we have found that the TrueVIEW reagent lifts off the tissue using TBS or HEPES buffer. Detergents are incompatible.
How long can I store the TrueVIEW working solution?
Once made up, the working solution of TrueVIEW can be stored either on the bench top or in the fridge (2-8C) fro about 48 hours (2 days) withut loss of activity or function. Following this time we would suggest discarding unused working solution and make fresh solution as required.
Is TrueVIEW effective against lipofuscin derived autofluorescence?
The working solution of TrueVIEW works in an electrostatic manner to greatly reduce or eliminate fluorescence in tissue sections induced through the use of an aldehyde based fixative. TrueVIEW is also effective at reducing fluorescence from tissue components such as collagen, elastin and red blood cells. TureVIEW does not work to reduce autofluorescence due to lipofuscin.
My tissue sections turned blue when I applied TrueVIEW. Is this supposed to happen?
Yes, the working solution is a blue color that does “stain” the tissue section blue. This indicates an active and appropriate chemical reaction is occurring. The blue stain on the section does not fluoresce and does not interfere with the immunofluorescence application.
What mounting media can I use with TrueVIEW?
Both TrueVIEW products, SP-8400 and SP-8500, are supplied with 2 mL of VECTASHIELD Vibrance antifade mounting media. We have found that the mounting media does play a crucial role in maintaining the high signal to noise ratio when using TrueVIEW. VECTASHIELD Vibrance is included therefore as a critical component of the kit and it is recommended to use the media supplied for optimal results. At this time we do not have sufficient data to confidently recommend the use of other vendors mounting media with TrueVIEW. Substitution of VECTASHIELD Vibrance with another mounting media may result in less than satisfactory results.
Why is the TrueVIEW™ Autofluorescence Quenching reagent applied after completion of the IF assay and not at the start of the procedure?
During product development, applying the TrueVIEW™ Quenching reagent at the end of our standard IF procedure yielded the most optimal reduction in autofluorescence signal. The reagent is retained on the tissue at the time of mounting, allowing for extended quenching action, with little to no effect on the specific fluorescent signal. However, in applications with very brief staining procedures, such as a primary antibody directly conjugated with a fluorophore, application of TrueVIEW™ Quenching reagent may be just as effective at the start of the procedure.
- Lectin Glycobiology
-
Glysite™ Scout Glycan Screening
Kits, Immunofluorescence
Can the biotinylated Lycopersicon esculentum (tomato) lectin be used for in vivo perfusion studies to trace blood vessels in mice?
The Lycopersicon esculentum (tomato) lectin has been widely reported as an effective blood vessel marker for in vivo vascular perfusion studies in rodent species. Investigators have primarily utilized one of the fluorophore conjugated tomato lectin formats to trace blood vasculature in normal and diseased animals via tail vein or intracardiac injection. However, the biotinylated format has also been used. It allows for flexibility in subsequent visualization by way of either fluorescence or enzyme-based methods. Published references are best source for procedural details. Examples of references where biotinylated tomato lectins have been applied via in vivo perfusion are featured below: Robertson, R.T., et al. (2014) Histochem. Cell Biol. 143(2) Thurston, G., et al. (1998) Am. J Pathol. 153(4):1099-1112
Can you provide an extinction coefficient for the lectins?
We can provide a protein absorbance value read at O.D. 280 nm for unconjugated lectins. This value represents a 0.1% (1 mg/mL) solution measured in a 1 cm cuvette. It should be noted that these values are percent solution extinction coefficients (ε percent), which are different from molar extinction coefficients.
How many tissue sections can I process with one Glysite™ Scout Glycan Screening Kit, Immunofluorescence?
Each Glysite Scout Glycan Screening Kit contains enough reagents for approximately 50 tissue samples per lectin, or up to 400 samples if using one lectin per tissue sample (8 lectins x 50 tissue samples).
How were the lectins selected for the Glysite™ Scout Glycan Screening Kit, Immunofluorescence?
The lectins were selected based upon their broad applicability and specificity to frequently cited glycan targets from literature sources, spanning fields of Cancer, Immunology, Metabolism, Inflammation, Virology, and Tumor Microenvironment, among others. They enable detection of all the major glycan motifs for the evaluation of glycan distribution in a target specimen.
How would I adapt the Glysite™ Scout Glycan Screening Kit, Immunofluorescence protocol to co-label with an antibody stain?
If co-labeling with a primary antibody, users should optimize each protocol separately to achieve maximum signal to noise ratios. Once this is complete, the two protocols can be combined, with the Glysite Scout Glycan Screening Kit protocol applied first, followed by the antibody staining protocol. Users should perform the proper controls to demonstrate that the reagents in each protocol are not cross–reacting with each other.
I have a fluorophore conjugated lectin from Vector Labs. It is supplied as a liquid concentrate. How to I apply this to a tissue section?
We offer lectins conjugated with a variety of fluorophores, including established haptens such as fluorescein and more contemporary fluorophores such as DyLight dyes. Regardless, these lectin fluorophore conjugates are all applied in a similar manner for cell and tissue staining applications. The fluorophore conjugated lectins can be applied to either FFPE specimens, or fixed frozen material. Once the specimen has been prepared (fixed, sectioned), placed onto a glass slide and brought to buffer (50-100 mM Tris, pH7.4, 150 mM NaCl), sections can be blocked with a high grade BSA or Carb-Free Blocker (SP-5040) for 20-30 min at R/Temp. The blcoking reagent is tipped off and then a working solution of the fluorophore conjugated lectin is applied for 30-60 min, R/Temp. To obtain a suitable working solution, we suggest taking an aliquot from the stock lectin concentrate supplied and diluting 5-20 ug/ml in Tris buffer. Following incuibation with the lectin, the specimen is washed 2x 2 min in buffer and then coverslipped with a suitabel antifade mounting media such as VECTASHIELD.
I purchased an agarose bound lectin from Vector Labs. Do you have a protocol outline on how this may be applied in a column format?
Our agarose lectin products are supplied as hydrated matrix solutions in amber glass bottles. The agarose (bead) material will settle and you will see two phases in the tube supplied. The upper phase is buffer. A column can be prepared in a commercial plastic device such as Bio-Rad Cat # 732-6008 or an inverted Pasteur pipet with glass wool lightly packed in the neck to retain the agarose. 1) Draw (pipet) the desired amount of settled agarose-lectin (gel) from the stock bottle into the prepared column and let the buffer drain by gravity.(Sometimes an air bubble in the column tip prevents flow; tapping the column should get the flow started). 2) Wash the gel with 10 column volumes of buffer, such as HBS (10 mM HEPES, 0.15 M NaCl, pH 7.5) and discard the flow through. 3) Place a collection vessel (e.g. glass test tube) under the column tip and apply the glycoprotein-containing solution.Allow the solution to drain through using gravity. We recommend against pushing or pulling the material through the column. Retain the flow through material until the desired binding has been confirmed. 4) After sample application, wash column with 2-3 column volumes of buffer (or until the absorbance at 280nm is reduced to a satisfactory level) to remove unbound materials before elution. 5) Place a fresh collection vessel under the column tip.Apply the eluting solution again letting gravity do the work of moving the solution over the column. Note that in some cases, several column volumes of eluting solution may be required to achieved adequate release of bound material. 6) Following elution, the column can be prepared for reuse by washing with 10 column volumes of buffer. 7) If the column is to be stored, equilibrate the column with buffer containing 0.08% sodium azide. Cover the column with a plastic wrap, or similar, to prevent desiccation and keep at 4 degrees Celsius. The column will be stable for many months when stored under these conditions.
I recently purchased a biotinylated lectin. The datasheet supplied with the lectin suggests including 0.1 mM Ca++as part of the recommended buffer to prepare a working solution. What should I specifically add, and why is this required?
From our experience we have found that some lectins require Ca++ to be present for optimal binding activity. We suggest using calcium chloride (CaCl2) to fortify working solutions and ensure a minimum level of Ca++ is meet. This may be particularly pertinent if using phosphate based buffers as diluents and storage solutions.
What are recommended conditions for using the agarose-lectin in chromatography?
The pH should be near neutral, the maximum pressure for packing the resin is 10 psi, and the maximum flow rate 3.5 ml/min
What are the advantages of using VECTASHIELD Vibrance® over VECTASHIELD® PLUS or other mounting media with Glysite™ Scout Glycan Screening Kit, Immunofluorescence?
VECTASHIELD Vibrance is an antifade medium for immunofluorescence applications with setting properties, which means the coverslip is immobilized after curing and does not require sealing. Additionally, slides can be stored at room temperature, enabling extended archiving time. VECTASHIELD PLUS does not have setting properties and remains molten, and slides must be stored at 2–8°C. Both VECTASHIELD Vibrance and VECTASHIELD PLUS have superior antifade/anti-photobleaching properties across the spectrum and are compatible with commonly used fluorophores.
What Glysite™ Scout Glycan Screening Kit, Immunofluorescence is right for me?
Each of the three Glysite Scout Glycan Screening Kits, Immunofluorescence contain the same lectins but with a different fluorescent streptavidin detection reagent, offering a choice of Dylight™ 488 (GSK-3000), Dylight™ 594 (GSK-2000), or Dylight™ 649 (GSK-1000). This allows users to choose the kit which best matches the capabilities of their fluorescent microscope and also provides flexibility for multiplexing with other fluorescent detection reagents they might already be using.
What is an appropriate blocking reagent to use in combination with lectin conjugates when applying to tissue sections?
Since lectins bind sugars and glycoproteins, it is recommended to avoid “standard” blocking reagents such as serum, milk derivatives (casein, non-fat dry milk) or similar solutions that may contain simple or complex carbohydrates. We suggest using product SP-5040, Carbo-Free Blocking Solution, to avoid potential interaction with sugars and reduce specific binding when using lectins.
What is the primary composition of the Vector Labs glycoprotein eluting solutions? Do any of these contain protein?
The glycoprotein eluting solutions are largely comprised of specific sugars and defined salts that are usually required to remove bound material from agarose conjugated lectins. These eluting solutions do not introduce harsh pH conditions or other factors that may affect the eluted glycoproteins or the lectin column. As such, we view these mixtures as being both eluting and regeneration solutions. None of these solutions contains protein.
What tissue types can I use with Glysite™ Scout Glycan Screening Kits, Immunofluorescence?
Virtually any tissue type and species are suitable for use with a Glysite Scout Glycan Screening Kit, Immunofluorescence, as glycans are ubiquitous in nature and in life. The kit has been validated on human and mouse tissue—including FFPE tissue, across a range of organs, including colon, lung, spleen, kidney, liver, pancreas, testes, heart, bladder, and uterus.
When do I need to consider antigen retrieval when applying these lectins to tissue sections?
The need for an antigen retrieval step in a staining protocol depends on multiple variables, including the target glycan and its location in the specimen, the glycan-binder, the type of tissue, and the method and length of fixation. Investigators should run controls with and without antigen retrieval to determine if this step is needed for their specimen.
Why do some of your sample images use the Vector® TrueVIEW® Autofluorescence Quenching Kit?
Tissue components such as red blood cells, elastin, and collagen are strongly fluorescent, particularly in FFPE sections, making it difficult to discern between relevant signal and background. Formalin fixation tends to induce a significant amount of fluorescence to a specimen. The Vector TrueVIEW Autofluorescence Quenching Kit provides a dramatic reduction of autofluorescence, allowing a clear view of the desired signal, so it was utilized as needed on the specimens represented in the images.
Why is Carbo-Free Blocking Solution used with Glysite™ Scout Glycan Screening Kit, Immunofluorescence?
The Carbo-Free Blocking Solution included in the Glysite Scout Glycan Screening Kit is virtually free of glycoproteins or other glycosylated molecules. This is critical since these kits detect glycans and would therefore bind to blocking reagents which contained glycosylated components. Users should not use other blocking solutions (e.g., milk derivatives, sera) as it may lead to high background.
How many tissue sections can I process with one Glysite™ Scout Glycan Screening Kit, Immunofluorescence?
Each Glysite Scout Glycan Screening Kit contains enough reagents for approximately 50 tissue samples per lectin, or up to 400 samples if using one lectin per tissue sample (8 lectins x 50 tissue samples).
How were the lectins selected for the Glysite™ Scout Glycan Screening Kit, Immunofluorescence?
The lectins were selected based upon their broad applicability and specificity to frequently cited glycan targets from literature sources, spanning fields of Cancer, Immunology, Metabolism, Inflammation, Virology, and Tumor Microenvironment, among others. They enable detection of all the major glycan motifs for the evaluation of glycan distribution in a target specimen.
How would I adapt the Glysite™ Scout Glycan Screening Kit, Immunofluorescence protocol to co-label with an antibody stain?
If co-labeling with a primary antibody, users should optimize each protocol separately to achieve maximum signal to noise ratios. Once this is complete, the two protocols can be combined, with the Glysite Scout Glycan Screening Kit protocol applied first, followed by the antibody staining protocol. Users should perform the proper controls to demonstrate that the reagents in each protocol are not cross–reacting with each other.
What are the advantages of using VECTASHIELD Vibrance® over VECTASHIELD® PLUS or other mounting media with Glysite™ Scout Glycan Screening Kit, Immunofluorescence?
VECTASHIELD Vibrance is an antifade medium for immunofluorescence applications with setting properties, which means the coverslip is immobilized after curing and does not require sealing. Additionally, slides can be stored at room temperature, enabling extended archiving time. VECTASHIELD PLUS does not have setting properties and remains molten, and slides must be stored at 2–8°C. Both VECTASHIELD Vibrance and VECTASHIELD PLUS have superior antifade/anti-photobleaching properties across the spectrum and are compatible with commonly used fluorophores.
What Glysite™ Scout Glycan Screening Kit, Immunofluorescence is right for me?
Each of the three Glysite Scout Glycan Screening Kits, Immunofluorescence contain the same lectins but with a different fluorescent streptavidin detection reagent, offering a choice of Dylight™ 488 (GSK-3000), Dylight™ 594 (GSK-2000), or Dylight™ 649 (GSK-1000). This allows users to choose the kit which best matches the capabilities of their fluorescent microscope and also provides flexibility for multiplexing with other fluorescent detection reagents they might already be using.
What tissue types can I use with Glysite™ Scout Glycan Screening Kits, Immunofluorescence?
Virtually any tissue type and species are suitable for use with a Glysite Scout Glycan Screening Kit, Immunofluorescence, as glycans are ubiquitous in nature and in life. The kit has been validated on human and mouse tissue—including FFPE tissue, across a range of organs, including colon, lung, spleen, kidney, liver, pancreas, testes, heart, bladder, and uterus.
When do I need to consider antigen retrieval when applying these lectins to tissue sections?
The need for an antigen retrieval step in a staining protocol depends on multiple variables, including the target glycan and its location in the specimen, the glycan-binder, the type of tissue, and the method and length of fixation. Investigators should run controls with and without antigen retrieval to determine if this step is needed for their specimen.
Why do some of your sample images use the Vector® TrueVIEW® Autofluorescence Quenching Kit?
Tissue components such as red blood cells, elastin, and collagen are strongly fluorescent, particularly in FFPE sections, making it difficult to discern between relevant signal and background. Formalin fixation tends to induce a significant amount of fluorescence to a specimen. The Vector TrueVIEW Autofluorescence Quenching Kit provides a dramatic reduction of autofluorescence, allowing a clear view of the desired signal, so it was utilized as needed on the specimens represented in the images.
Why is Carbo-Free Blocking Solution used with Glysite™ Scout Glycan Screening Kit, Immunofluorescence?
The Carbo-Free Blocking Solution included in the Glysite Scout Glycan Screening Kit is virtually free of glycoproteins or other glycosylated molecules. This is critical since these kits detect glycans and would therefore bind to blocking reagents which contained glycosylated components. Users should not use other blocking solutions (e.g., milk derivatives, sera) as it may lead to high background.
- Bioconjugation
- General Questions
- Streptavidin Magnetic Beads
- Protein–Oligo / Ab–Oligo Conjugation
- HyNic/4FB
Are SoluLINK catalog products normally shipped at ambient temperature?
Yes, we ship all SoluLINK catalog products at ambient temperature. We did set our expiration dates on the conservative side, knowing that the products could spend a significant amount of time at ambient temperature during transit.
Can I centrifuge streptavidin magnetic beads without affecting their binding properties?
It is very important that the beads not be exposed to high centrifugal force, as this will cause them to form a tight clump that will be very difficult to disperse. Alternating between vigorous vortexing and sonication is recommended to prevent the beads from forming a tight clump. Note: we do not have much data on using a centrifuge to pellet beads, as a magnet is typically used.
Can I modify less than 100 ug of antibody using the Antibody-Oligo Conjugation Kit?
Unfortunately, there is not a reliable way to modify the Antibody-Oligo Conjugation Kit to process samples other than what the kit was designed. For instance, each Zeba column is pre-equilibrated in a specific/proprietary buffer, so it would not be possible to process more than one sample at a time.
Can you help me choose between NanoLINK and MagnaLINK streptavidin magnetic beads? I’m having trouble figuring out which one is right for my application.
NanoLINK beads are smaller and polydisperse, but have a higher binding capacity than MagnaLINK beads (note: NanoLINK 1.0 µm Streptavidin Magnetic Beads have the highest biotin binding capacity on the market).
MagnaLINK Streptavidin Magnetic Beads are monodisperse and suitable for flow cytometry to create a FSC/SSC profile, in addition to other applications requiring a tight size distribution.
Could you please provide instructions for preparing 2-Sulfobenzaldehyde (S-2005-100) and 2-Hydrazinopyridine (S-2002-100) from lyophilized form?
Create a 0.5 mM solution of either 2HP or 2SB in 100 mM MES buffer and adjust the pH to 5.00 +/- 0.05 using 1M HCl or 1M NaOH as needed. After adjusting the pH, bring the solution to the final volume with water (or MES buffer, depending on how the MES solution was made). It is recommended to sterile filter the solutions using a 0.22 µm syringe filter. For storage, the solutions must be protected from light and stored at 4°C. Under these conditions the solutions should be stable for at least 6 months.
During HyNic/protein MSR quantification, the pH of the MES buffer is 5 when diluting 2-sulfobenzaldehyde (2-SBA) during the colorimetric assay. Could you explain why an acidic pH is ideal?
Hydrazone formation is acid catalyzed and proceeds much more rapidly at an acidic pH. We chose MES, pH 5.0, as it is a good buffer at this pH — PBS/TBS/HEPES do not have good buffering capacity at pH 5.0 as it is too far away from the isoelectric point of the proteins.
For B-9007-105K, why you would need to perform buffer exchange on the protein if you have reconstituted it in the 1x modification buffer?
The purpose of the buffer exchange step is to remove amine contaminants, such as Tris or glycine, from the protein. These contaminants may interfere with NHS ester labeling, which targets primary amine groups. Some samples are so excessively contaminated that two buffer exchanges may be required before proceeding with biotinylation.
For the antibody-oligo conjugation kit, can I use a smaller protein to conjugate the oligo – instead of an antibody?
We recommend against it. Using a different protein with a molecular weight other than 150 kDa, and/or an amount other than 100 µg, will likely cause the reaction to fail.
How do I store purified antibody-oligo conjugates?
Antibody-oligo conjugates may be diluted and stored at 4°C in PBS, pH 7.2, plus 0.05% sodium azide and 1 mM EDTA. The PBS we use is 10 mM sodium phosphate, 150 mM sodium chloride, pH 7.20. Other PBS formulations may also be used successfully. The azide and EDTA can be omitted if necessary for live cell work and/or for IHC with enzymes that require metal ions for full functionality or for enzymes that are not compatible with azide such as HRP.
How do I track bioconjugation of protein to oligonucleotide? Is there a traceable/quantifiable means to assess progress in conjugating my protein?
By virtue of the hydrozone linkage, SoluLINK’s HyNic-4FB conjugate bond is spectrophotometrically UV traceable at 354 nm. The modifications of both the HyNic linker on the protein and the 4FB linker on the oligonucleotide are quantifiable using colorimetric assays. The kit contains all the reagents necessary to determine the number of HyNic linkers per probe (i.e., the Molar Substitution Ratio) for both protein and oligo.
How long can I store VECTABOND® working solution once diluted in acetone?
VECTABOND Reagent is supplied as a 7 ml concentrate that is diluted in acetone immediately prior to use. Once diluted in acetone, as per the instructions for use, it is recommended that the working solution be used as soon as possible. Storage of this working solution is not practical beyond a few hours as the chemicals will start to break down and the solution will turn a yellowish color. Once this has happened, slide coating will not be optimal and the working solution should be discarded.
What is the concentration (number of beads/ml) for NanoLINK and MagnaLINK streptavidin magnetic beads?
NanoLINK = 3.406 X 1010 beads/mL
MagnaLINK = 5.458 X 108 beads/mL
What is the difference between Protein-Oligo Conjugation Kit – (S-9011) and the Antibody – Oligonucleotide All-in-One Conjugation Kit (A-9202)?
The Protein-Oligo Conjugation Kit (S-9011) was designed with more flexibility in mind to allow conjugation of any protein greater than about 20 kDa, to an oligonucleotide. No purification material is included in this kit because there are so many different proteins that can be conjugated. This kit also allows for a variable amount of protein to be coupled (from about 50µg up to 650µg).
The Antibody – Oligonucleotide All-in-One Conjugation Kit (A-9202) was designed specifically for simple and reproducible coupling of an oligo to a fixed amount of antibody (100µg). All kit components have been optimized around this amount, which reduces the number of calculations involved and reduces variability in the process. This kit does include purification so the final conjugate is very pure (i.e., no free antibody or oligo remains in the final product), which allows kit usage in very sensitive assays. Since this kit is designed for antibodies, purification is accomplished using a resin that binds to all mammalian IgG antibody isotypes. Yields from this kit are typically 30-60% based on the amount of starting antibody. Longer oligos (60-mers and up) tend to have a lower yield (in the 20-40% range) due to steric hinderance of the antibody binding to the resin.
What is the purpose of Sulfo versions of the S-HyNic and S-4FB linkers?
Sulfo versions of the linkers are typically used for delicate biomolecules which cannot tolerate any DMF. Stock solutions prepared in DMF are stable for a few days at -20 °C if prepared with anhydrous DMF. The Sulfo linkers can be dissolved in water or buffer instead of DMF, but they must be used immediately.
Why do I see precipitates in a protein-protein conjugate?
Precipitation is often caused by over-modification of one or both proteins. When the HyNic- and 4FB-modified proteins are conjugated, a precipitate forms due to polymerization. There are other factors in play also, such as the concentration of the two proteins in the conjugation reaction, incubation time, aniline concentration, and isoelectric point of the proteins. If good conjugation results are achieved at small scale when the antibodies are at 2 mg/mL each, it may be advisable to dilute them to 1 mg/mL during a large-scale reaction. The conjugation buffer should be slightly acidic for best results (~pH 6), however the reaction will proceed within the range of pH 4.5 – 7.5.
Would molar substitution ratio (MSR) be able to determine HyNic-antibody coupling efficiency? Why is MSR important for HyNic and 4FB-modified molecules?
The HyNic MSR is an important benchmark to ensure the antibody has received enough linkers without being over-modified. A range of 3 – 6 HyNic groups per antibody is usually best. To determine the antibody binding efficiency to the beads, a Bradford or BCA assay of the bead supernatant (unbound antibody) is performed to determine the amount of antibody that did not couple to the beads. This assay should be performed after an overnight incubation of the HyNic-modified antibody with the 4FB beads to ensure enough time has elapsed to allow for complete conjugation. The conjugation should take place with slow and gentle end-over-end mixing to keep the beads from settling.
Are SoluLINK catalog products normally shipped at ambient temperature?
Yes, we ship all SoluLINK catalog products at ambient temperature. We did set our expiration dates on the conservative side, knowing that the products could spend a significant amount of time at ambient temperature during transit.
Could you please provide instructions for preparing 2-Sulfobenzaldehyde (S-2005-100) and 2-Hydrazinopyridine (S-2002-100) from lyophilized form?
Create a 0.5 mM solution of either 2HP or 2SB in 100 mM MES buffer and adjust the pH to 5.00 +/- 0.05 using 1M HCl or 1M NaOH as needed. After adjusting the pH, bring the solution to the final volume with water (or MES buffer, depending on how the MES solution was made). It is recommended to sterile filter the solutions using a 0.22 µm syringe filter. For storage, the solutions must be protected from light and stored at 4°C. Under these conditions the solutions should be stable for at least 6 months.
For B-9007-105K, why you would need to perform buffer exchange on the protein if you have reconstituted it in the 1x modification buffer?
The purpose of the buffer exchange step is to remove amine contaminants, such as Tris or glycine, from the protein. These contaminants may interfere with NHS ester labeling, which targets primary amine groups. Some samples are so excessively contaminated that two buffer exchanges may be required before proceeding with biotinylation.
Can I centrifuge streptavidin magnetic beads without affecting their binding properties?
It is very important that the beads not be exposed to high centrifugal force, as this will cause them to form a tight clump that will be very difficult to disperse. Alternating between vigorous vortexing and sonication is recommended to prevent the beads from forming a tight clump. Note: we do not have much data on using a centrifuge to pellet beads, as a magnet is typically used.
Can you help me choose between NanoLINK and MagnaLINK streptavidin magnetic beads? I’m having trouble figuring out which one is right for my application.
NanoLINK beads are smaller and polydisperse, but have a higher binding capacity than MagnaLINK beads (note: NanoLINK 1.0 µm Streptavidin Magnetic Beads have the highest biotin binding capacity on the market).
MagnaLINK Streptavidin Magnetic Beads are monodisperse and suitable for flow cytometry to create a FSC/SSC profile, in addition to other applications requiring a tight size distribution.
What is the concentration (number of beads/ml) for NanoLINK and MagnaLINK streptavidin magnetic beads?
NanoLINK = 3.406 X 1010 beads/mL
MagnaLINK = 5.458 X 108 beads/mL
Can I modify less than 100 ug of antibody using the Antibody-Oligo Conjugation Kit?
Unfortunately, there is not a reliable way to modify the Antibody-Oligo Conjugation Kit to process samples other than what the kit was designed. For instance, each Zeba column is pre-equilibrated in a specific/proprietary buffer, so it would not be possible to process more than one sample at a time.
For the antibody-oligo conjugation kit, can I use a smaller protein to conjugate the oligo – instead of an antibody?
We recommend against it. Using a different protein with a molecular weight other than 150 kDa, and/or an amount other than 100 µg, will likely cause the reaction to fail.
How do I store purified antibody-oligo conjugates?
Antibody-oligo conjugates may be diluted and stored at 4°C in PBS, pH 7.2, plus 0.05% sodium azide and 1 mM EDTA. The PBS we use is 10 mM sodium phosphate, 150 mM sodium chloride, pH 7.20. Other PBS formulations may also be used successfully. The azide and EDTA can be omitted if necessary for live cell work and/or for IHC with enzymes that require metal ions for full functionality or for enzymes that are not compatible with azide such as HRP.
How do I track bioconjugation of protein to oligonucleotide? Is there a traceable/quantifiable means to assess progress in conjugating my protein?
By virtue of the hydrozone linkage, SoluLINK’s HyNic-4FB conjugate bond is spectrophotometrically UV traceable at 354 nm. The modifications of both the HyNic linker on the protein and the 4FB linker on the oligonucleotide are quantifiable using colorimetric assays. The kit contains all the reagents necessary to determine the number of HyNic linkers per probe (i.e., the Molar Substitution Ratio) for both protein and oligo.
What is the difference between Protein-Oligo Conjugation Kit – (S-9011) and the Antibody – Oligonucleotide All-in-One Conjugation Kit (A-9202)?
The Protein-Oligo Conjugation Kit (S-9011) was designed with more flexibility in mind to allow conjugation of any protein greater than about 20 kDa, to an oligonucleotide. No purification material is included in this kit because there are so many different proteins that can be conjugated. This kit also allows for a variable amount of protein to be coupled (from about 50µg up to 650µg).
The Antibody – Oligonucleotide All-in-One Conjugation Kit (A-9202) was designed specifically for simple and reproducible coupling of an oligo to a fixed amount of antibody (100µg). All kit components have been optimized around this amount, which reduces the number of calculations involved and reduces variability in the process. This kit does include purification so the final conjugate is very pure (i.e., no free antibody or oligo remains in the final product), which allows kit usage in very sensitive assays. Since this kit is designed for antibodies, purification is accomplished using a resin that binds to all mammalian IgG antibody isotypes. Yields from this kit are typically 30-60% based on the amount of starting antibody. Longer oligos (60-mers and up) tend to have a lower yield (in the 20-40% range) due to steric hinderance of the antibody binding to the resin.
What is the purpose of Sulfo versions of the S-HyNic and S-4FB linkers?
Sulfo versions of the linkers are typically used for delicate biomolecules which cannot tolerate any DMF. Stock solutions prepared in DMF are stable for a few days at -20 °C if prepared with anhydrous DMF. The Sulfo linkers can be dissolved in water or buffer instead of DMF, but they must be used immediately.
Why do I see precipitates in a protein-protein conjugate?
Precipitation is often caused by over-modification of one or both proteins. When the HyNic- and 4FB-modified proteins are conjugated, a precipitate forms due to polymerization. There are other factors in play also, such as the concentration of the two proteins in the conjugation reaction, incubation time, aniline concentration, and isoelectric point of the proteins. If good conjugation results are achieved at small scale when the antibodies are at 2 mg/mL each, it may be advisable to dilute them to 1 mg/mL during a large-scale reaction. The conjugation buffer should be slightly acidic for best results (~pH 6), however the reaction will proceed within the range of pH 4.5 – 7.5.
During HyNic/protein MSR quantification, the pH of the MES buffer is 5 when diluting 2-sulfobenzaldehyde (2-SBA) during the colorimetric assay. Could you explain why an acidic pH is ideal?
Hydrazone formation is acid catalyzed and proceeds much more rapidly at an acidic pH. We chose MES, pH 5.0, as it is a good buffer at this pH — PBS/TBS/HEPES do not have good buffering capacity at pH 5.0 as it is too far away from the isoelectric point of the proteins.
Would molar substitution ratio (MSR) be able to determine HyNic-antibody coupling efficiency? Why is MSR important for HyNic and 4FB-modified molecules?
The HyNic MSR is an important benchmark to ensure the antibody has received enough linkers without being over-modified. A range of 3 – 6 HyNic groups per antibody is usually best. To determine the antibody binding efficiency to the beads, a Bradford or BCA assay of the bead supernatant (unbound antibody) is performed to determine the amount of antibody that did not couple to the beads. This assay should be performed after an overnight incubation of the HyNic-modified antibody with the 4FB beads to ensure enough time has elapsed to allow for complete conjugation. The conjugation should take place with slow and gentle end-over-end mixing to keep the beads from settling.
- What is dPEG®?
- Case Study
What is dPEG®?
The term “dPEG®” is Vector Laboratories’s trademarked acronym for “discrete polyethylene glycol” or “discrete PEG”. The “discrete” portion of the dPEG® trademark indicates single molecular weight PEG technology. Like traditional PEGs, our products contain an amphiphilic backbone of repeating ethylene oxide units. However, traditional PEGs are not single compounds. Vector Laboratories invented and manufactures these monodisperse PEG products using our proprietary synthetic and purification processes. Read more.
What is the difference between monodisperse PEGs and discrete PEGs?
Both Vector Laboratories’s dPEG® products and monodisperse PEGs have no dispersity (Đ = 1). See Figure 1. Monodisperse PEGs are typically separated from polymerization mixtures by various modes of chromatography to provide a single chain length and molecular weight, thereby avoiding complications resulting from dispersity. While some commercially available monodisperse PEGs have sizes up to 48 ethylene oxide units (MW >2,000), the vast majority of monodisperse PEGs have 12 or fewer units (MW ~500). In our patented production process, dPEG® products are prepared via standard organic chemistry methodology and not by polymerization techniques, thereby providing a single molecule with a defined and specified chain length, molecular weight, and purity. This easily scalable process is routinely applied to provide linear dPEG® products up to 2 kDa and branched dPEG® products up to 16 kDa.
The terms monodisperse PEG and discrete PEG overlap in meaning, but there are significant differences between the two terms. These differences have consequences for drug development and characterization. Describing our dPEG® products as “monodisperse,” though not inappropriate, connotes incorrect concepts with respect to our compounds. “Monodisperse” implies that either:
1) The compounds are single compounds that were made in a one-pot polymerization process and then purified from the polymeric mixture, which our compounds are not; or,
2) There is only one compound of uniform functionality, size, and shape formed from a polymeric process, which is purely a theoretical concept.
We prefer the term “discrete” since our products are synthesized in a step-wise fashion as single molecular weight compounds from pure starting materials using standard organic methodology. Thus, the terminology associated with polymer chemistry is not completely accurate. Therefore, a monodisperse PEG is a single compound prepared via a polymerization process and separated from the mixture, while a dPEG® is a single molecule of specified length and molecular weight prepared via defined stepwise reactions.
How are dPEG® products named?
Our linear products are typically named as R1-dPEG®x-R2, where R1 and R2 denote functional, reactive, or protective groups and x indicates the number of oxygen atoms in the spacer unit. We do this to simplify the naming of the compounds. Our catalog shows the exact structure of each compound.
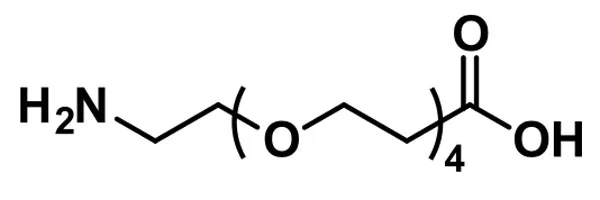
FIgure 2: Product number QBD-10244, Amino-dPEG®24-acid. The abbreviated chemical structure as shown on the website.
For example, product number QBD-10244, amino-dPEG®4-acid, has an amino group on one end, four (4) ethylene oxide units, and a carboxylic acid group on the other end. See Figure 2. Similarly, product number QBD-10198, NHS-dPEG®12-biotin, has a biotin label on one end, twelve oxygens in the discrete PEG chain, and an amine-reactive N-hydroxysuccinimide (NHS) ester on the opposite end.
What are your capabilities?
Vector Laboratories has been making dPEG® products for more than 20 years. During this time, we have greatly expanded our capabilities and product lines. We offer a broad range of highly pure dPEG® modifiers, linkers, and spacers ranging in molecular weights from 200 to about 2,300 Daltons for linear compounds and up to 16,000 Daltons for branched compounds. The market has hitherto been limited to smaller monodisperse PEGs (~1200 Da or smaller) or medium-sized conventional disperse PEGs (2,000-3,400 Da up to 20,000 Da or higher). Even these have not been sufficiently exploited, mostly due to a lack of commercial availability stemming from synthetic and purification challenges, the limited functionality of the PEGs, and irreproducible lot-to-lot variability.
Our dPEG® products are prepared via a robust and highly reproducible process and can incorporate a large range of functionalities. Thus, we have the flexibility to produce any dPEG® product (including custom products) on scales ranging from milligrams (mg) to multi-Kilograms (Kg) with purity suitable for research, diagnostic, and therapeutic applications. Our commercial-scale reactors allow us to achieve these larger quantities of our products. While we do not operate a cGMP facility we have cGMP manufacturing partners to support both commercial cGMP and non-cGMP production. We are available for customer audits, with appropriate policies and procedures in place to guarantee reproducible results. From initial product development through market release and beyond, we help our customers produce a safe, reliable, superior product.
For a complete list of our product offerings, please take a look at our online catalog. We also offer custom syntheses to develop molecules tailored to specific needs, so if you do not see what you are looking for please contact us and allow us to leverage our experience in the field to assist you.
Do dPEG® products affect the immune system?
A major reason for using PEG in the development of biotherapeutic agents is that it is classified as non-immunogenic. Indeed, it has long been known that PEG shields molecules from the immune system. This is called the “stealth effect” of PEG.
In recent years, some reports in the scientific literature claim to observe immune system responses – including the formation of anti-PEG antibodies – to PEGylated biomolecules. For example, large dispersed PEG products have been shown to be immunogenic when conjugated to highly immunogenic proteins. These reports have not gone unchallenged, because there remain many unanswered questions about this phenomenon.
Research has shown that the size and type of terminal group of a traditional PEG make a difference in the immune system response to PEG-biomolecule conjugates. Additionally, PEG architecture, chain length, and surface coating density may influence the potential immune response. Furthermore, recruitment of certain so-called “stealth” proteins to the PEGylated surface appears to suppress immune system responses to PEGylated biomolecules has been demonstrated.
Vector Laboratories’s dPEG® products have been shown to prevent opsonization by the immune system, thus demonstrating the stealth effect of PEG. Moreover, an immunogenic response to biomolecules modified with our dPEG® products has not been demonstrated. Possibly, the short, discrete chain length of dPEG® molecules possess suitable architecture and provide sufficient surface coating density to enable dPEG®-modified biomolecules to evade immune system responses.
Why use dPEG® products instead of traditional PEG linkers and spacers?
Although the historical scientific literature demonstrates the utility of high molecular weight PEGs, we do not offer comparable linear dPEG® products (beyond dPEG®48). Negative chemical and physical properties are observed with the larger linear PEGs. For example, large linear, dispersed PEGs have been shown to greatly diminish the potency and efficacy of the biomolecules to which they are conjugated (e.g., antibodies, enzymes). Furthermore, in PEGylated lipid nanoparticles (LNPs) a phenomenon exists that is known as the “PEG dilemma.” The PEG dilemma is this: as the amount of surface PEGylation with high molecular weight PEG increases on an LNP, the stability and serum half-life of the LNP increase, but the binding affinity, potency, and efficacy of the conjugated biomolecule decrease. The PEG dilemma’s effects include (but are not limited to) the following:
- Poor cell internalization;
- Poor endosomal escape;
- Reduced targeting efficiency; and,
- Diminished potency and efficacy of conjugated biomolecules.
Vector Laboratories’s dPEG® products sharply limit or even avoid the negative effects that arise from large, linear, traditional PEGs. First, our linear PEG products are much smaller than traditional PEG products. As the case studies above demonstrate, in many cases, short, linear, dPEG® linkers and spacers perform better than traditional, large, linear PEG linkers and spacers. Second, for higher molecular weight dPEG® products, we circumvent the intrinsic, negative properties of large, linear, traditional PEGs by synthesizing branched dPEG® constructs. These dPEG® products use shorter chains (4 to 24 ethylene oxide units per chain) containing 3 to 9 branches that are assembled into a variety of architectures. These unique constructs provide our customers with high molecular weight dPEG® products (>15 kDa) as single molecular weight compounds with discrete chain lengths.
Thus, we recommend dPEG® products over traditional PEG products for three reasons.
- The single molecular weight and discrete chain length of dPEG® products give our customers complete control over the design and assembly of macromolecular architectures. See Figure 1, above.
- The single molecular weight and discrete chain length of dPEG® products (again, see Figure 1) simplifies the analysis of products that incorporate dPEG®
- Our dPEG® products avoid the problems associated with large, linear, traditional PEGs, including loss of binding affinity, loss of potency and efficacy, and the “PEG dilemma” by using short, linear, dPEG® products or by using high-molecular-weight branched dPEG® In many published studies, dPEG® products outperform traditional PEG products.
These reasons are “the dPEG® difference” that cause our products to stand out over traditional, linear PEG products. To see a positive difference in your research or product development, incorporate our dPEG® constructs in your diagnostic, therapeutic, or theranostic products.
Are dPEG® products as good as, or better than, high molecular weight PEG products?
There are several reasons to use Vector Laboratories’s dPEG® products instead of a traditional, disperse PEG product.
Vector Laboratories’s dPEG® Products
- Each dPEG® product is a single compound.
- When a dPEG® is conjugated to another molecule, there is a single reaction product.
- Because there is a single reaction product, analysis of the final product purity is simplified.
- A simplified analysis of the final product purity saves time and uses fewer resources, thus saving money.
Traditional, Disperse PEG Products
- Each traditional PEG product is a complex mixture of PEG chains.
- When a traditional PEG is conjugated to another molecule, there are many different reaction products arising from PEG dispersity.
- Because there are multiple PEG chain lengths in the final product, the analysis is complex and time-consuming;
- A complex, time-consuming analysis can be quite expensive in terms of labor and resources.
Many of our customers come to us thinking that they need a large PEG in order to improve water solubility, eliminate aggregation, reduce non-specific binding, or impart reduced antigenicity/immunogenicity to their target. The prevailing thought in the bioconjugation community has been that “bigger is better.” This is not always the case, though. Rather the optimal PEG size depends on the specific application. Some case studies are illustrative.
Do your dPEG® products contain other PEG homologs?
Our dPEG® products are synthesized from high-purity starting materials using standard organic chemistry techniques in a series of multi-step reactions, above. Each dPEG® product has a specified size, molecular weight, architecture, and functionality and is characterized in the same manner as small molecules. Thus, the only PEG in our dPEG® products is the one described in the name and number, and the purity that we report is not based on average molecular weight, but on a specific molecular weight. This was shown in a 2009 study by Alister C. French, et al., where a dPEG®24 product from Vector Laboratories was tested and found to have Đ= 1.000058 at 96% purity.
Conversely, traditional PEGs prepared via polymerization contain other homologs, and PEG used in the past typically had a Đ of up to 1.2. Years of research and development have driven this value lower so that the currently accepted standard for PEG reagents is Đ of 1.01 – 1.05 for mPEGs less than 5 kDa in size to more than 1.1 for PEGs greater than 50 kDa in size. Moreover, there is no way to guarantee that the size and molecular weight distribution profile of one lot of dispersed PEG will match the profile of subsequent lots of dispersed PEG.
As the requirements for the approval of new PEG conjugates become more stringent, the trend towards a narrower range of molecular weights is expected to continue. For instance, the first two PEG conjugates brought to the market in the early 1990s, Adagen® (pegademase bovine) and Oncaspar® (pegaspargase), needed only demonstrate the reproducibility of conjugation. In the case of both drugs, the PEG was treated “as an excipient that contributed to the pharmacokinetic properties of the molecule. When Pegasys® (Peginterferon alfa-2a) and Peg-Intron® (Peginterferon alfa-2b) were introduced ten years later, regulatory agencies required more thorough characterization of each product, including the number of PEGs conjugated to the protein, characterization of each isomer, specific conjugation sites, and stability of the conjugated protein, among many other items. Although the conjugation sites of these therapeutic products were characterized, they still employed disperse PEGs. This gave rise to a population of drug conjugates with different biological properties among the members of the population. By contrast, the use of a monodisperse PEG was shown clearly to simplify the characterization of three model proteins as compared to results achieved with PEGylating the model proteins with disperse PEGs. As further research demonstrates the impact the PEG size and shape have on both chemistry and biology of PEG-conjugates, monodisperse and discrete PEGs will become increasingly important.
In what applications are dPEG® products used?
Our products are used currently for multiple applications, including the following:
- linkers for payloads/warheads in antibody-drug conjugates (ADCs) and other targeting molecules (Fab, PDCs, );
- performance enhancing modifiers of pharmacokinetic, pharmacodynamic, and biodistribution properties of therapeutic, diagnostic, and theranostic biomolecules and small molecule pharmaceuticals;
- surface modification of gold, iron, iridium, carbon nanotubes, and other biocompatible surfaces;
- assembly of both targeted and non-targeted micelles, liposomes, dendrimers, and other nanoparticles;
- functionalization and “stealthing” of primary and secondary antibodies; and,
- affinity tags, fluorescent dyes, and radiolabels for diagnostic applications.
Our dPEG® products convey the beneficial properties of traditional PEGs, such as increased water solubility, reduced aggregation, increased hydrodynamic volume, and reduced immunogenicity, but they do so while limiting the complications that arise from using disperse PEGs and allow for the rational design of dPEG® conjugates and analysis of their structure-activity relationships.
Are dPEG® products patented?
To protect our clients’ interests, Vector Laboratories patented the processes for the production of dPEG® constructs (see US Patents #7,888,536 and 8,637,711), allowing them the freedom to operate. We also have several patents pending on compositions of matter related to dPEG® constructs. Numerous companies worldwide distribute our products, but we manufacture all of our products in our facilities in Plain City, Ohio.
Is a large linear PEG always better?
It should be noted that in the development of Pegasys® (INF-α2a) and PEG-Intron® (INF-α2b), a branched 40 kDa PEG was found to provide the optimum therapeutic properties for Pegasys®, while a linear 12 kDa PEG provided optimum therapeutic properties for PEG-Intron®, thereby demonstrating that large linear PEGs are not always the best choice for development.
What solvents work best with dPEG® products?
Our dPEG® products are amphiphilic, which means that they are soluble in both water and some organic solvents. The amphiphilicity of any given dPEG® product may shift depending on the reactive, protective, or functional groups that sit at the ends of the dPEG® chains. Thus, some dPEG® products may prefer organic solvent over water, while others may prefer water over organic solvent. However, PEG chains hydrogen-bond three (3) water molecules per oxygen atoms in the chain. Thus, a dPEG® product always has some water solubility, though it may be rather small.
“Green” and sustainable chemistry increases in importance to scientists yearly. Numerous reviews address green and sustainable chemistry.
Table 1: Useful Solvents for dPEG® Products
| Solvent Name | Green(ish) Solvent? | Boiling Point (°C) at 101.308 kPa | Known Characteristics, Issues & Cautions |
| Actonitrile | Y/N | 82 | Dissolves most dPEG® products, but see text for exceptions; not universally regarded as a green solvent. |
| Acetone | Y | 56 | Dissolves many dPEG® products; incompatible with amines and oxy-amine (R-O-NH2) dPEG® products (reaction). |
| N,N-dimethylacetamide (DMAC) | N | 165 | Dissolves most dPEG® products readily; water miscible; high boiling point; possible reproductive toxicity. |
| N,N-dimethylformamide (DMF) | N | 153 | Dissolves most dPEG® products readily; water miscible; high boiling point; possible reproductive toxicity. |
| Dimethylsulfoxide (DMSO) | Y | 189 | Dissolves most dPEG® products readily, but may be incompatible with some products; water miscible; high boiling point. |
| Ethanol | Y | 78 | Good solvent for many dPEG® products; cannot use with dPEG® products containing active esters (reaction) |
| Ethyl acetate | Y | 77 | Usefulness restricted to dPEG® products ≤dPEG®8; even in this range, it does not dissolve all dPEG® products. |
| Methanol | N | 65 | Good solvent for many dPEG® products; cannot use with dPEG® products containing active esters (reaction); known toxicity issues. |
| 2-Propanol | Y | 82 | Does not dissolve all dPEG® products; can be used to dissolve active esters (no reaction). |
| Tetrahydrofuran | Y | 66 | Usefulness restricted to dPEG® products ≤dPEG®12 if anhydrous conditions are required; for larger dPEG® products, up to 30 vol% water may be required to effect complete dissolution. |
| Water | Y | 100 | Dissolves most dPEG® products readily, but see text for exceptions. Hydrolyzes active esters at pH >7. |
Acetonitrile
Most dPEG® products dissolve readily in acetonitrile. The notable exceptions are the amino-dPEG®-acids from dPEG®12 and smaller. Product numbers QBD-10244, QBD-10067, and QBD-10277 are insoluble in acetonitrile. Product number QBD-10287, amino-dPEG®12-acid, is sparingly soluble in acetonitrile. For these products, methanol and water are the preferred solvents.
Acetonitrile dissolves dPEG® products containing active esters (NHS esters and TFP esters). However, acetonitrile readily absorbs water from the atmosphere, and the presence of water in the solvent may hydrolyze the active esters. Vector Laboratories strongly recommends drying acetonitrile by standing it over 3Å molecular sieves for 24 – 48 hours before use.
Acetonitrile is water-miscible. Reactions with biologics such as proteins, peptides, glycans, and nucleic acids preferentially take place in aqueous buffer. For dPEG® products that hydrolyze or have limited solubility in water, dry acetonitrile can be used to make a stock solution of those dPEG® products. An aliquot of the stock solution is added to the reaction buffer at the time of the reaction.
CAS number: 75-05-8
PubChem CID: 6342
ChemSpider ID: 6102
UNII: Z072SB282N
Acetone
Acetone dissolves many dPEG® products and is relatively benign for environmental health and safety (EHS) considerations. However, it is incompatible with amines (reacts to form a Schiff base) and oxyamine-containing products (reacts to form an oxime). Acetone is highly hygroscopic and should be dried over molecular sieves before using it.
CAS number: 67-64-1
PubChem CID: 180
ChemSpider ID: 175
UNII: 1364PS73AF
Dimethylacetamide and Dimethylformamide
Dimethylacetamide (DMAC) and dimethylformamide (DMF) dissolve most dPEG® products. DMAC and DMF are water-miscible and dry readily upon standing for 24 – 48 hours over 3Å molecular sieves. These two solvents readily absorb water from the atmosphere. Consequently, drying over molecular sieves is necessary before using these solvents with dPEG® products containing active esters.
DMF tends to degrade over time and form free amines that can react, for example, with active esters of dPEG® acids. When using with dPEG® products, DMF should be of the highest purity and “fresh” (out of a newly opened bottle) or recently distilled. DMAC does not degrade over time. Therefore, we at Vector Laboratories prefer DMAC over DMF.
Dimethylacetamide
CAS number: 127-19-5
PubChem CID: 31374
ChemSpider ID: 29107
UNII: JCV5VDB3HY
Dimethylformamide
CAS number: 68-12-2
PubChem CID: 6228
ChemSpider ID: 5993
UNII: 8696NH0Y2X
Dimethylsulfoxide
Dimethylsulfoxide (DMSO) is a well-known, widely used green solvent. When pure, DMSO is a clear, colorless, water-miscible, polar aprotic solvent. It dissolves nearly all of Vector Laboratories’s dPEG® products. DMSO has a high boiling point (189°C at 101.3 kPa/760 Torr) and, thus, is difficult to remove from a reaction mixture. Stock solutions of dPEG® products in DMSO find frequent use in reactions with biological products in aqueous reaction media. When used to dissolve dPEG® compounds containing active esters, DMSO must be dried over 3Å molecular sieves before use and then handled under dry conditions because it is highly hygroscopic.
CAS number: 67-68-5
PubChem CID: 679
ChemSpider ID: 659
UNII: YOW8V9698H
Ethanol
Ethanol is a relatively environmentally friendly compound that can be a useful solvent for many dPEG® products. However, ethanol is unsuitable for dissolving products containing NHS or TFP active esters. The active esters exchange with ethanol in solution, resulting in a non-functional product. Ethanol is hygroscopic; therefore, drying ethanol over molecular sieves before use may be necessary.
CAS number: 64-17-5
PubChem CID: 702
ChemSpider ID: 682
UNII: 3K9958V90M
Ethyl Acetate
Ethyl acetate has limited usefulness with dPEG® products. Nevertheless, for dPEG® products of size dPEG®8 or smaller ethyl acetate may prove useful, especially when the end functional groups are hydrophobic. Ethyl acetate is relatively benign to the environment.
CAS number: 141-78-6
PubChem CID: 8857
ChemSpider ID: 8525
UNII: 76845O8NMZ
Methanol
Methanol dissolves most dPEG® products. However, like ethanol, do not use methanol with dPEG® compounds containing NHS or TFP active esters. Moreover, unlike ethanol, methanol has well-known toxicity issues. Methanol is the preferred organic solvent for amino-dPEG®-acids ≤dPEG®12 (see the text under acetonitrile for more information).
CAS number: 67-56-1
PubChem CID: 887
ChemSpider ID: 864
UNII: Y4S76JWI15
2-Propanol
The solvent 2-propanol (also called isopropanol or isopropyl alcohol) has limited use with dPEG® products. It is a somewhat viscous, hygroscopic solvent. Although it does not have a high boiling point compared to DMAC, DMF, or DMSO, it still can be challenging to remove all 2-propanol from a reaction mixture.
CAS number: 67-63-0
PubChem CID: 3776
ChemSpider ID: 3644
UNII: ND2M416302
Tetrahydrofuran
Tetrahydrofuran (THF) is another solvent with limited usefulness. If anhydrous conditions are required, THF alone is not able to completely dissolve products that are longer than a dPEG®12 chain. For these longer dPEG® products, up to 30 volume% water may be required with THF to get the product to dissolve fully.
CAS number: 109-99-9
PubChem CID: 8028
ChemSpider ID: 7737
UNII: 3N8FZZ6PY4
Water
Water is the ultimate green solvent, and water-solubility is one of the characteristics for which dPEG® products are renowned. Nevertheless, two cautions apply when using water as a solvent for dPEG® products. First, users should be aware that water hydrolyzes active esters to carboxylic acids. The hydrolysis occurs at and above pH 7, with the hydrolytic rate increasing with pH. Second, dPEG® products with strongly hydrophobic functional groups on the dPEG® chain have poor-to-no solubility in water. For example, product number QBD-11362, DBCO-dPEG®4-TFP ester, has a hydrophobic functional group on each end of the dPEG® chain. Consequently, it has very poor water solubility; DMAC, DMSO, and acetonitrile are recommended alternatives to water for this product.
CAS number: 7732-18-5
PubChem CID: 962
ChemSpider ID: 937
UNII: 059QF0KO0R
Are there other useful solvents?
Undoubtedly, more solvents than the ones listed above dissolve our dPEG® products. The solvents listed above are the ones that Vector Laboratories’s scientists use on a semiregular-to-regular basis. They know these solvents and their limitations, and these are the ones that we recommend to our customers. If you find a solvent outside of this list that you think we should put on the list, please contact us and let us know. We love to hear our customers’ thoughts and suggestions on how better to use our dPEG® products.
Pegasys® and Peg-Intron®
Pegasys® (Peginterferon alfa-2a; Schering-Plough) and Peg-Intron® (Peginterferon alfa-2b; Hoffman-LaRoche) are two PEGylated protein (cytokine) drugs developed from Interferon α2A (Pegasys®) and Interferon α2B (Peg-Intron®), both of which are approximately 19 kDa proteins, differing by only one amino acid at position 23 of the sequence. Both Pegasys® and Peg-Intron® effectively treat Hepatitis C, a potentially life-threatening infection caused by the Hepatitis C virus (HCV). In developing both drugs, initial screening was conducted using disperse 5 kDa PEG. In both drugs, the isoforms generated using the 5 kDa PEG were found to be unsatisfactory in comparison to the unmodified protein.
During the development of Pegasys®, the 40 kDa conjugate showed substantially modified PK parameters resulting in dramatically improved clinical efficacy compared to the unmodified IFN α2a. Although the mono-PEGylated conjugate only retained 7% of the parent’s in-vitro activity, the improved in-vivo profile provided a blockbuster drug and first-line treatment for hepatitis C.
In the case of Peg-Intron®, however, a mono-PEGylated IFN-a2B for the treatment of hepatitis C achieved the desired effect with a linear 12 kDa disperse PEG. In this case, the conjugate retained 28% of the unmodified IFN-a2B in vitro activity, and although the increase in systemic exposure is modest when compared to the 40 kDa conjugate, the balance between PK and PD provided another blockbuster drug for the treatment of hepatitis C.
Somavert®
An example of an even smaller disperse PEG conveying the desired properties can be seen with Somavert®, a PEGylated form of the 22 kDa human growth hormone for the treatment of acromegaly. In this case conjugation of four to six 5 kDa disperse PEGs resulted in a 28-fold decrease in binding affinity, but this was offset by a 400-fold increase in serum half-life, providing a second-line treatment for acromegaly.
These demonstrated successes with 40 kDa, 12 kDa, and 5 kDa disperse PEGs illustrate the point that PEG size alone is not as important as the proper balance of PK and PD in order to achieve the desired clinical effect, as well as reproducibility of manufacturing processes. In fact, while a 5 kDa PEG failed with Pegasys® it provided the desired profile with Somavert®.
Organophosphorus Hydrolase
In addition, a branched dPEG® from Vector Laboratories (M.W. 2,420 Da) was found to improve the pharmacokinetics and immunogenicity profiles of the enzyme organophosphorus hydrolase.
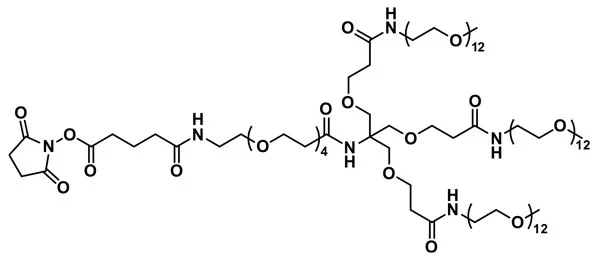
Figure 4: Vector Laboratories product number QBD-10401, NHS-dPEG®4-(m-dPEG®12)3 ester (molecular weight 2,420 Daltons) improved the pharmacokinetic and immunogenic profiles of the enzyme organophosphorus hydrolase.
124I Tumor Imaging
In another example, a three-branched dPEG® with a molecular weight of 4,473 Da with three negative charges (from the ends of the three branches) was conjugated to an antibody fragment (Fab’) and labeled with 124I for tumor imaging. This construct (see, Figure 5, below) was found to provide superior tumor retention and imaging quality and opened up the possibility for simpler analytical testing in drug discovery studies.
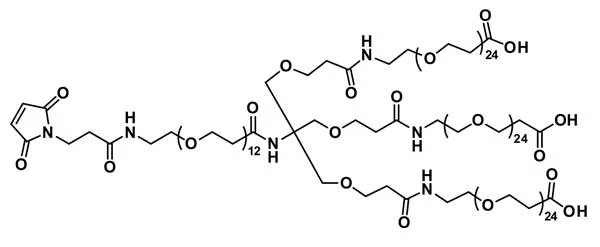
Figure 5: Vector Laboratories product number QBD-11451, MAL-dPEG®12-Tris(-dPEG®24-acid)3, a thiol-reactive, three-branched, negatively charged dPEG® construct with a molecular weight of 4,473 Daltons was demonstrated in animal testing to have superior tumor retention, leading to improved imaging quality.
Amphotericin B
For small-molecule drugs, even smaller PEG conjugates provide dramatic improvement. One striking example is the radical modification of properties that occurs when a small, discrete PEG of only eight (8) ethylene glycol units is attached to the water-insoluble, toxic antifungal drug Amphotericin B.

Briefly, Amphotericin B, a polyene macrolide antifungal drug, is the first-line, “gold standard” treatment for fungal infections and for the disease known as leishmania. Nevertheless, Amphotericin B is poorly soluble in water, is difficult to administer, and exhibits long-term systemic toxicity. Liposomal Amphotericin B delivery systems, with and without PEG, are used clinically, but systemic toxicity remains. PEG-Amphotericin B conjugates improve the water solubility of Amphotericin B, but until recently, relatively large PEGs of 5 kDa or above have been used, and the results were not impressive.
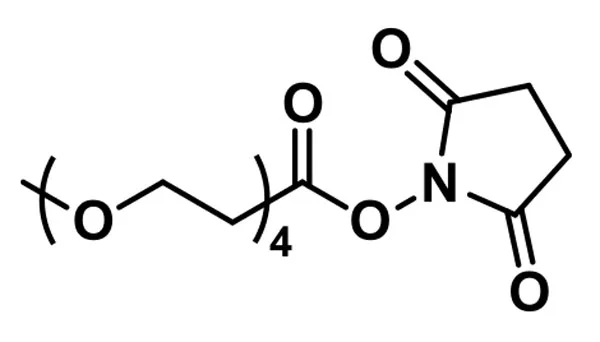
Figure 7 Product number QBD-10211, m-dPEG®4-NHS ester, used by Tan et al. to modify the antifungal drug Amphotericin B.
In 2016, two papers demonstrated that short, dPEG® products conjugated to Amphotericin B substantially alter the physical and pharmacological properties of the drug, including increased water solubility and decreased toxicity, without loss of antifungal efficacy. In both papers, the dPEG® was linked to the single free amine of Amphotericin B. The paper by Tan, et al., used a methoxy-terminated dPEG®4, m-dPEG®4-NHS ester; molecular weight, 333.3 Daltons; dPEG® molecular weight 220.3, Daltons). In contrast, Halperin and colleagues used Vector Laboratories product QBD-10995, Fmoc-dPEG®8-NHS ester; molecular weight, 760.8 Daltons; dPEG® molecular weight, 441.5 Daltons), followed by removal of the Fmoc protecting group to expose the terminal amine.
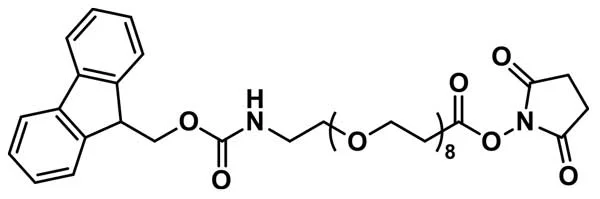
Figure 8 Product number QBD-10995, Fmoc-N-amido-dPEG®8-NHS ester, used by Halperin et al to modify Amphotericin B.
The research by Tan, et al., allowed the formation of a liposome-like suspension that remained dispersed in water even at high concentrations. In vitro toxicity was reduced considerably, and the product remained efficacious in killing two species of fungus that are pathogenic to humans. In the paper by Halperin, et al., after removal of the Fmoc protecting group from the dPEG®8, the Amphotericin B-dPEG®8 conjugates demonstrated water solubility, sharply reduced in vivo toxicity in mice, and efficacious in vitro and in vivo killing of pathogenic fungi. Taken together, these two papers show that it is possible to develop a clinically useful therapeutic product using only small, discrete PEG products. Moreover, because the dPEG® moiety is a single molecular weight compound, the conjugated product can be characterized fully. With a dispersed PEG, the characterization of the several conjugates formed in the reaction would be a time-consuming challenge.
Antibody-Drug Conjugates (ADCs)
In the areas of bioconjugation, ADCs, diagnostics, and surface modification the focus is not on large PEG size as much as on hydrophilic linkers of defined and consistent lengths. Both hetero- and homobifunctional dPEG® linkers with as few as four ethylene oxide units have been shown to impart beneficial properties to the resulting conjugates, and the ability to employ varying dPEG® lengths has allowed the optimization of the desired properties.
For example, researchers at Immunogen used hydrophilic likers for the construction of antibody-drug conjugates. They found that dPEG® linkers with as few as four ethylene oxide units were able to provide antibody-maytansinoid conjugates that doubled typical drug-antibody ratios (DARs), were much more potent than lower DARs, did not aggregate, and retained antibody affinity.
James R. Prudent and co-workers at Centrose Pharma developed a new class of antibody-drug conjugates (ADCs) called an “extracellular antibody-drug conjugate.” (EDC) to deliver a toxic payload to a specific extracellular site that is proximal to an extracellular target of an antibody. The researchers evaluated linkers between the antibody and the drug. The linkers contained 0, 2, 12, 24, and 36 ethylene glycol units in the dPEG® chain. They found that the dPEG® linkers all enhanced delivery of the cardiac glycoside scillarenin β-L-aminoxyloside to tumor cells expressing dysadherin, a protein marker associated with metastatic cancer, that was targeted by the monoclonal antibody to which the payload was conjugated. The EDC with the dPEG®36 linker (approximately 1.9 kDa linear dPEG® from Vector Laboratories.) worked both in vitro and in vivo in mice. The in vivo performance of the EDC exceeded the performance of Rituximab. This work also shows that effective delivery of a cytotoxic payload with reduced hydrophobicity does not require a large PEG such as a 5 or 10 kDa (or larger) PEG. Instead, the highly specific therapeutic effect was delivered using a linker that is less than 2.2 kDa in size.
Molecular Beacons
In another example, researchers at the NIH synthesized a series of molecular beacons for video imaging of protease expression in a matrix metalloprotease-overexpressing tumor-bearing mouse model. In order to achieve true, real-time imaging and superior signal-to-noise ratios a probe must have the proper balance between in vivo stability and sensitivity. The researchers conjugated a series of dPEG® products (n = 4, 12, 24, 48) to their probe to study this. While no significant in vitro differences were observed, the dPEG®12 conjugate showed significant in vivo enhancements including the onset of activation, signal-to-noise ratio, and tumor selectivity. This suggests that targeting of specific proteases can be tested and optimized by conjugating low molecular weight dPEG® products to various probes.
Gold Nanoparticles
Two additional examples can be cited to show the utility of short-chain, single molecular weight PEG products.
For the first example, in 2010 and 2011, researchers at Vanderbilt University in the lab of David E. Cliffel showed that the substitution of short-chain thiol-PEG®4-alcohol or thiol-dPEG®4-acid provided superior, non-immunogenic protection of gold nanoparticles against opsonization in vivo.
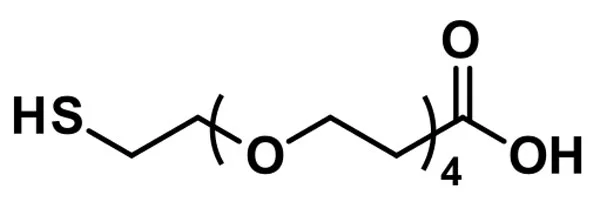
Figure 9: Product number QBD-10247, Thiol-dPEG®4-acid.
Galanin (neuropeptide)
In this example, Vector Laboratories product number QBD-10339, m-dPEG®24-acid, was site-specifically conjugated to a derivative of the neuropeptide galanin, which has analgesic effects in the peripheral nervous system. This prevented galanin from crossing the blood-brain barrier, but it concomitantly enhanced the peptide’s analgesic effects in the peripheral nervous system. Please note that product number QBD-10339 has been replaced by product number QBD-11289, m-dPEG®25-acid.

