What Is dPEG®?
What is PEGylation?
The benefits of PEGylation include increased water solubility, increased hydrodynamic volume, decreased immunogenicity, and extended circulation in vivo in the bloodstream due to reduced renal clearance. In addition to reducing renal clearance, PEGylation also often modifies the biodistribution and pharmacokinetics of therapeutic, theranostic, and diagnostic molecules.
Traditional PEGs
dPEG® Products Are Different
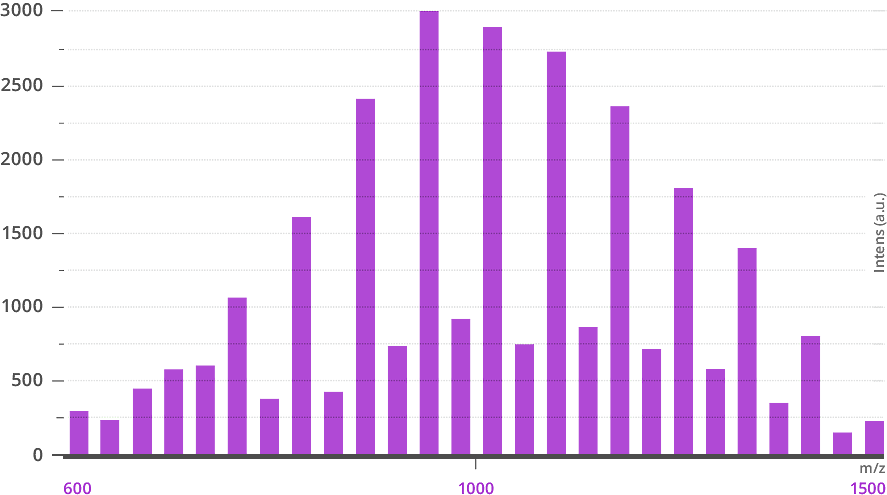
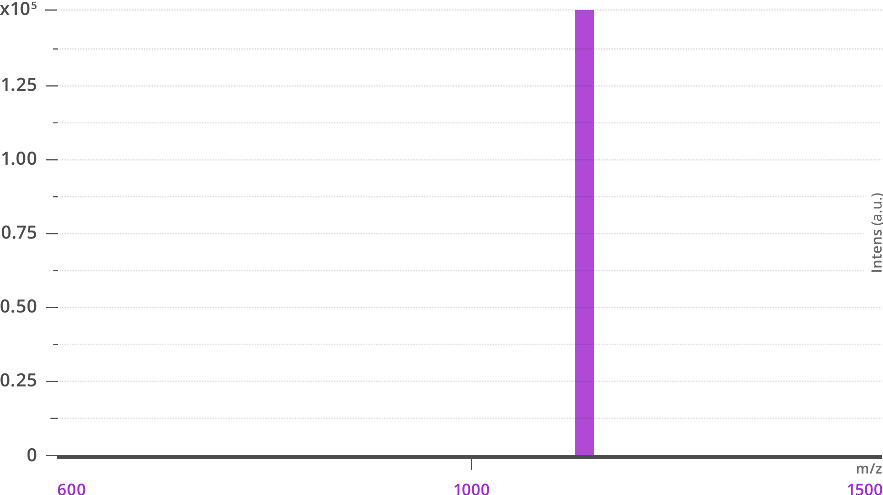
Side-by-side mass spectra of traditional polyethylene glycol (PEG) and a dPEG® of equivalent mass from Vector Laboratories

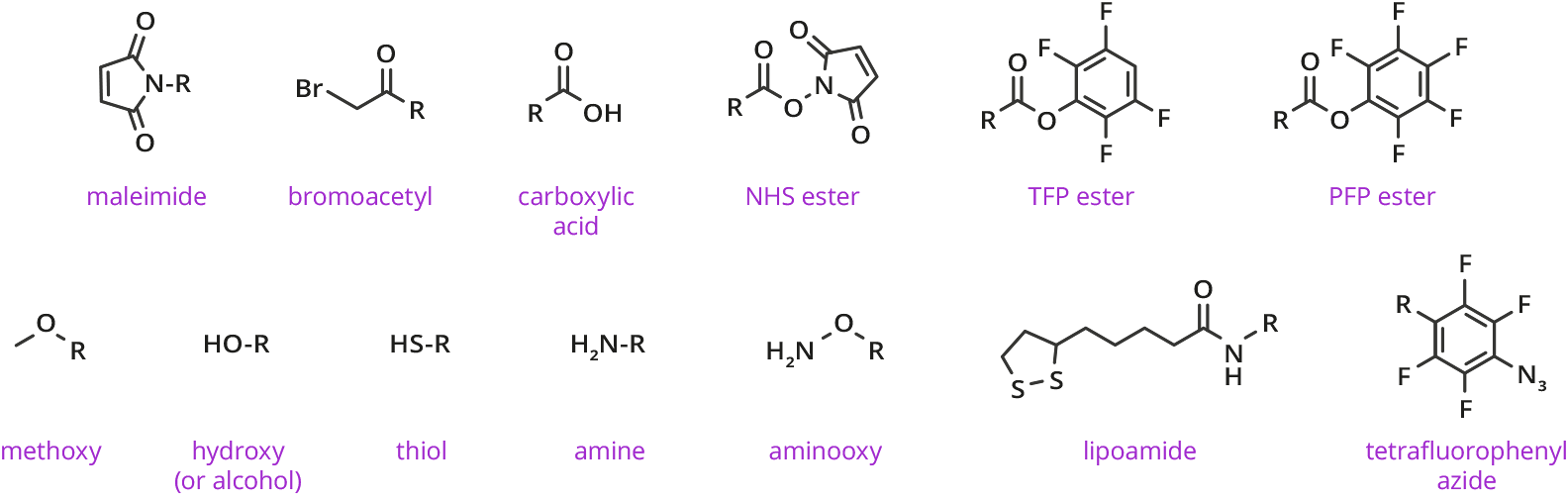
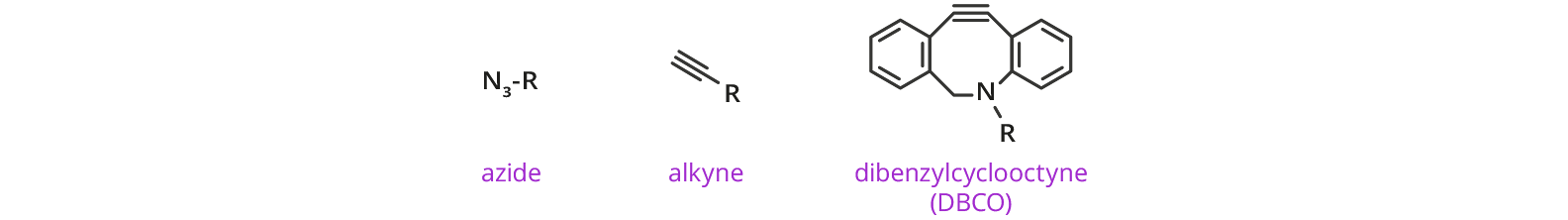
A Wide Variety of Functional Groups and Architectures for dPEG® Products
Functional/Reactive Groups for dPEG® Products
Click Chemistry Functional Groups with dPEG® Linkers/Spacers
Labeling Groups with dPEG® Linkers/Spacers
Other labels are also available!
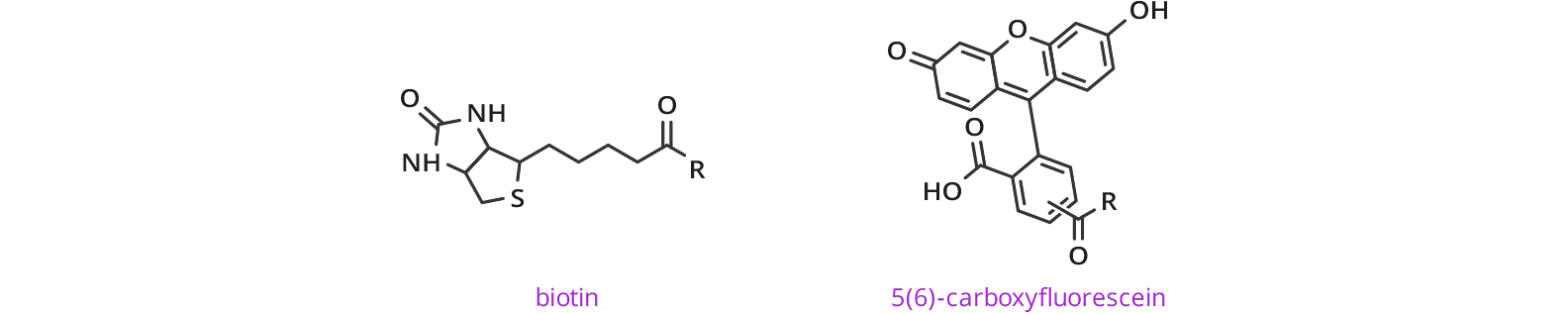
DOTA dPEG® Products with Various Functional Groups
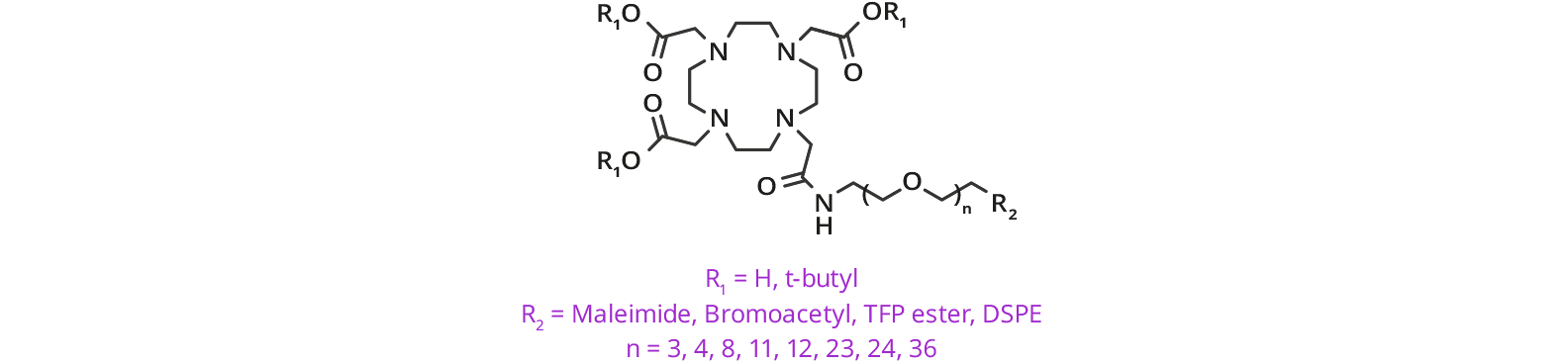
Protective Groups for dPEG® Products
Linker Architectures
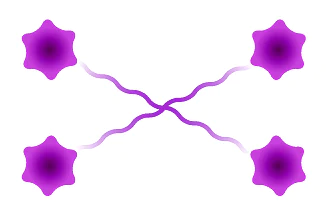
Homobifunctional
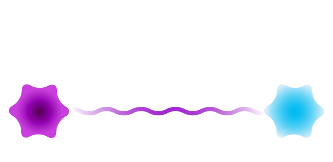
Heterobifunctional
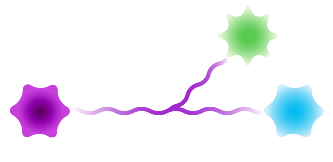
Sidewinder™
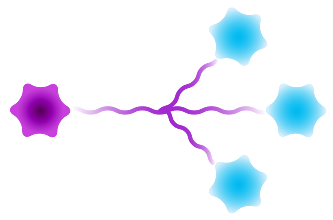
Branched
Tetramer
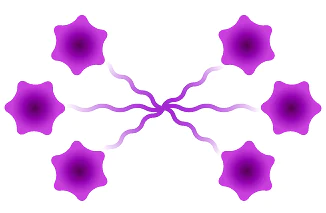
Hexamer
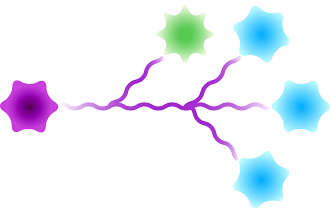
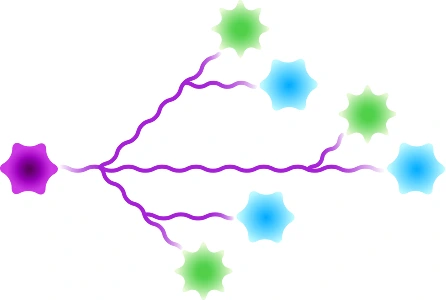
Scaffolded
Figure 3: Available architectures for dPEG® products. Purple starburst is reactive group to attach to the antibody, green – reactive group to attach to the payload/trigger, blue – end capping. Branched dPEG® products can have three (3) or nine (9) branches. Our Sidewinder™ products are a new class of bioconjugation linker that offers flexibility of controlling the distance of a payload from the antibody while optimizing hydrophilicity through the orthogonal PEG arm. The BodyArmor® product architecture is similar to the Sidewinder, but includes additional orthogonal dPEG® strands.
References
- The term “amphiphilic” means that the compound is soluble in both water (or aqueous buffer) and organic solvents. All PEG products that do not contain hydrophobic substituents are soluble in water and in a variety of organic solvents. The addition of hydrophobic groups to PEG reduces the water solubility of some PEG products.
- Flory, P. J. Molecular Size Distribution in Ethylene Oxide Polymers. J. Am. Chem. Soc. 1940, 62, 1561−1565. [ACS Publications]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F. R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170-2243. [ACS Publications]
- Gilbert, R. G.; Hess, M.; Jenkins, A. D.; Jones, R. G.; Kratochvíl, P.; Stepto, R. F. T. Dispersity in polymer science (IUPAC Recommendations 2009). Pure Appl. Chem. 2009, 81, 351–353. [De Gruyter]. See also, Stepto, R. F. T. Erratum. Pure Appl. Chem. 2009, 81, 779. [De Gruyter]
- Veronese, F. M.; Mero, A.; Pasut, G. Protein PEGylation, Basic Science and Biological Applications. In PEGylated Protein Drugs: Basic Science and Clinical Applications; Veronese, F. M., Ed.; Milestones in Drug Therapy; Birkhäuser Basel: Basel, 2009; pp 11–31. [Springer]
- Jevsevar, S; Kunstejl, M; Porekar, V.G. PEGylation of therapeutic proteins. Biotechnology Journal 2010, 5(1) 113-128. DOI: [Wiley]
- For example, dextran with Đ=2 is considered “low dispersity” today. Previously dextran had even higher dispersity than that. See, Pasut, G. Polymers for Protein Conjugation. Polymers 2014, 6(1), 160–178. [MDPI]

