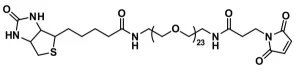
Biotin-dPEG®3-MAL, product number 10201, consists of biotin on one end of a dPEG®3 linker with a maleimidopropyl moiety (maleimide ring connected to propionic acid) on the other end. The flexible, short-chain, discrete PEG (dPEG®) linker imparts water solublity to conjugates formed with it. The maleimide group reacts with free thiols to form stable thioether linkages.
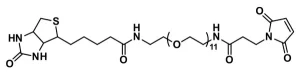
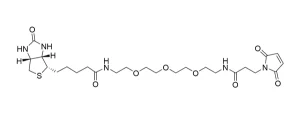
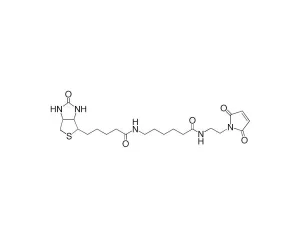
Biotin-dPEG®3-MAL, product number 10201, is one of three products containing biotin on one end of a dPEG® linker and a maleimidopropyl moiety on the other. For QBD-10201, the linker is a dPEG®3 product. The flexible, short-chain, discrete PEG (dPEG®) linker imparts water solubility to conjugates formed with it. The maleimide moiety is a popular reactive group for conjugating to free thiol groups in biomolecules and other sulfhydryl compounds. Maleimide groups can be conjugated to free thiol groups in the pH range 6.5 – 7.5, forming a stable thioether linkage.
This product has proven helpful in various applications that utilize the high streptavidin-biotin binding affinity. Such applications include
FRET-based assays
probes of protein topology
protein labeling
the creation of biotinylated, dPEG®-modified nucleosides
different types of high-throughput screening procedures
the construction of biomimetic gold-and-silica nanoparticles, and
the construction of self-assembling quantum dots.
To use Biotin-dPEG®3-MAL, dissolve the product in a dry solvent and add it to an aqueous compound containing the free thiol. The water-miscible solvent N,N’-dimethylacetamide (DMAC) dried over 3Å molecular sieves is an excellent solvent for this purpose. For biomolecules (e.g., antibodies), care should be taken to use a minimal amount of organic solvent so as not to denature the biomolecule or cause salt precipitation.
| Unit Size | 50 mg |
|---|---|
| Molecular Weight | 597.73; single compound |
| Chemical formula | C₂₇H₄₃N₅O₈S |
| CAS | 525573-22-2 |
| Purity | > 98% |
| Spacers | dPEG® Spacer is 21 atoms and 24.9 Å |
| Shipping | Ambient |
| Typical solubility properties (for additional information contact Customer Support) | Methylene chloride, DMAC or DMSO. |
| Storage and handling | -20°C; Always let come to room temperature before opening; be careful to limit exposure to moisture and restore under an inert atmosphere; stock solutions can be prepared with dry solvent and kept for several days (freeze when not in use). dPEG® pegylation compounds are generally hygroscopic and should be treated as such. This will be less noticeable with liquids, but the solids will become tacky and difficult to manipulate, if care is not taken to minimize air exposure. |
Greg T. Hermanson, Bioconjugate Techniques, 3rd Edition, Elsevier, Waltham, MA 02451, 2013, ISBN 978-0-12-382239-0; See Chapter 18, Discrete PEG Reagents, pp. 787-821, for a full overview of the dPEG® products.
Solid Phase 4’-Phosphopantetheinylation: Fungal Thiolation Domains are Targets for Chemoenzymatic Modification, Deirdre Stack, Aisling Frizzell, Karen Tomkins, and Sean Doyle. Bioconjugate Chem. 2009, 20 (8), pp 1514–1522. July 23, 2009. DOI: 10.1021/bc900071j.
Manipulation of Carrier Proteins in Antibiotic Biosynthesis, James J. La Clair, Timothy L. Foley, Tracy R. Schegg, Conor M. Regan, and Michael D. Burkart. Chemistry & Biology. 2004. 11 (2), pp 195-201. February 2004. DOI: 10.1016/j.chembiol.2004.02.010.
Biomimetic approach to the formation of gold nanoparticle/silica cor/shell structures and subsequent bioconjugation, Sung Min Kang, Kyung-Bok Lee, Dong Jin Kim and Insung S Choi. Nanotechnology. 2006, (17), pp. 4719–4725. September 1, 2006. DOI: 10.1088/0957.
Probing the Topological Tolerance of Multimeric Protein Interactions: Evaluation of an Estrogen/Synthetic Ligand for FK506 Binding Protein Conjugate. Terry W. Moore Jillian R. Gunther, and John A. Katzenellenbogen. Bioconjugate Chem. 2010, 21 (10), pp 1880–1889. October 4, 2010. DOI: 10.1021/bc100266v.
Exploration of Dimensions of Estrogen Potency Parsing Ligand Binding and Coactivator Binding Affinities. M. Jeyakumar, Kathryn E. Carlson, Jillian R. Gunther, and John A. Katzenellenbogen. Journal of Biological Chemistry. 2011 (286), pp. 12971-12982. February 14, 2011. DOI: 10.1074/jbc.M110.205112.
Monitoring a Coordinated Exchange Process in a Four-Component Biological Interaction System: Development of a Time-Resolved Terbium-Based One Donor/Three-Acceptor Multi-Color FRET System. Sung Hoon Kim, Jillian R. Gunther, and John A. Katzenellenbogen. JACS. 2010 132 (13) pp 4685-4692. April 7, 2011. DOI:10.1021/ja100248q.
Diarylpropionitrile (DPN) Enantiomers: Synthesis and Evaluation of Estrogen Receptor β-Selective Ligands. Vincent M. Carroll, M. Jeyakumar, Kathryn E. Carlson, and John A. Katzenellenbogen. J. Med. Chem. 2012, 55 (1) pp 528–537. November 28, 2011. DOI: 10.1021/jm201436k.
Solid Phase 4′-Phosphopantetheinylation: Fungal Thiolation Domains are Targets for Chemoenzymatic Modification.Deirdre Stack, Aisling Frizzell, Karen Tomkins, and Sean Doyle. Bioconjugate Chem. 2009, 20 (8) pp 1514–1522. July 23, 2009. DOI: 10.1021/bc900071j.
Attachment of hydrogel microstructures and proteins to glass via thiol-terminated silanes. Jeong Hyun Seo, Dong-Sik Shin, Priam Mukundan, Alexander Revzin. Colloids and Surfaces B: Biointerfaces. 2012, 98, pp 1–6. October 1, 2012. doi.org/10.1016/j.colsurfb.2012.03.025.
A Set of Time-Resolved Fluorescence Resonance Energy Transfer Assays for the Discovery of Inhibitors of Estrogen Receptor-Coactivator Binding. Jillian R. Gunther, Yuhong Du, Eric Rhoden, Iestyn Lewis, Brian Revennaugh, Terry W. Moore, Sung Hoon Kim, Raymond Dingledine, Haian Fu and John A. Katzenellenbogen. Journal of Biomolecular Screening. 2009, 14 (2) pp # 181-195. February 4, 2009. DOI: 10.1177/1087057108329349.
Structural characterization and functionalization of engineered spider silk films. Kristina Spieß, Stefanie Wohlrab and Thomas Scheibel. Soft Matter. 2010, 6 (17) pp 4168-4174. June 16, 2010. DOI: 10.1039/b927267d.
Enzyme-independent, orientation-selective conjugation of whole human complement C3 to protein surfaces. Daniel A. Mitchell, Rebecca Ilyas, Alister W. Dodds, Robert B. Sim. Journal of Immunological Methods. 2008, 337 (1) pp 49-54. June 9, 2008. DOI: 10.1016/j.jim.2008.05.011.
Colorimetric multiplexed immunoassay using specific aggregation of antigenic peptide-modified luminous nanoparticles. Toshihiro Ihara, Yasunori Mori, Takaaki Imamura, Motoko Mukae, Shojiro Tanaka, Akinori Jyo. Science Direct. 2006, 578 (1) pp 11-18. May 4, 2006. DOI: 10.1016/j.aca.2006.04.066.
An Anti-Insulin-like Growth Factor I Receptor AntibodyIs a Potent Inhibitor of Cancer Cell Proliferation. Erin K. Maloney, Jennifer L. McLaughlin, Nancy E. Dagdigian. Cancer Research. 2003, 63 pp 5073-5083. August 15, 2003. DOI:
5’-Sulfhydryl-Modified RNA: Initiator Synthesis, in Vitro Transcription, and Enzymatic Incorporation. Lei Zhang, Lele Sun, Zhiyong Cui, Robert L. Gottlieb and Biliang Zhang. Bioconjugate Chem.2001, 12 (6) pp 939–948. October 16, 2001. DOI: 10.1021/bc015504g.
Sugar-Binding Proteins from Fish: Selection of High Affinity “Lambodies” That Recognize Biomedically Relevant Glycans. Xia Hong, Mark Z. Ma, Jeffrey C. Gildersleeve, Sudipa Chowdhury, Joseph J. Barchi, Jr., Roy A. Mariuzza,∥ Michael B. Murphy, Li Mao and Zeev Pancer. ACS Chem. Biol. 2013, 8 pp 152−160. October 2, 2012. doi.org/10.1021/cb300399s.
High-Throughput Screening of Small Molecules Identifies Hepcidin Antagonists. Eileen Fung, Priscilla Sugianto, Jason Hsu, Robert Damoiseaux, Tomas Ganz, and Elizabeta Nemeth. Mol Pharmacol. 2013, 83 (3) pp 681-690. January 4, 2013. DOI.org/10.1124/mol.112.083428.
Self-Assembled Quantum Dot-Bioconjugates: Characterization and Use for Sensing Proteolytic Activity. Igor L. Medintz1, Thomas Pons, Kim E. Sapsford, Philip E. Dawson and Hedi Mattoussi. Proc. of SPIE. 2008, 6945. April 15, 2008. DOI: 10.1117/12.782174.
Interleukin-2 signalling is modulated by a labile disulfide bond in the CD132 chain of its receptor. Clive Metcalfe, Peter Cresswell and A. Neil Barclay. Open Biology. 2012, 2 (1). January 11, 2012. DOI: 10.1098/rsob.110036.
Electrochemical Template Synthesis of Multisegment Nanowires: Fabrication and Protein Functionalization. Bridget Wildt, Prashant Mali, and Peter C. Searson. Langmuir. 2006, 22 (25), pp 10528–10534. September 8, 2006. 10.1021/la061184j.
Targeted delivery of a photosensitizer to Aggregatibacter actinomycetemcomitans biofilm. Suci P, Kang S, Gmür R, Douglas T, Young M.. Antimicrobial Agents and Chemotherapy. 2010, 54 (6) pp 2489-2496. April 12, 2010. 10.1128/AAC.00059-10.
Applicable patents and legal notices are available at legal notices.
Stay in the Loop. Join Our Online Community
Products
Ordering
About Us
Application
Resources

©Vector Laboratories, Inc. 2025 All Rights Reserved.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Privacy Statement
How do I Request a Quote?
To request a quote for products: