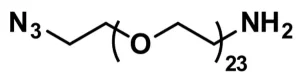
Azido-dPEG®3-amine, product number QBD-10522, is a carbonyl-reactive, biocompatible crosslinking reagent that can participate in multiple types of reactions. The product consists of an azide group and an amine group on opposite ends of a single molecular weight, discrete polyethylene glycol (dPEG®) chain. Alkynes react with the azido group in metal-catalyzed or strain-promoted click chemistry to yield triazole derivatives. Moreover, the azide group functions as a masked amine that reduces to a primary amine via a suitable reducing agent. Furthermore, Azido-dPEG®3-amine can participate in a Staudinger ligation with appropriate phosphine derivatives to create a water-soluble product.
Several patents and scientific publications specifically cite the use of Azido-dPEG®3-amine. The amphiphilic dPEG® product adds hydrodynamic volume and imparts water solubility to any compound to which it is conjugated. Also, because the terminal azide group functions in multiple different types of reactions, Azido-dPEG®3-amine can act as either a heterobifunctional, click chemistry crosslinker or a monoprotected homobifunctional crosslinking reagent.
The published uses of Azido-dPEG®3-amine include the following:
Detection of SK2 Channels on Hippocampal Neurons
Preparation of probes uses to study sickle cell disease;
Construction of tunable supramolecular assemblies;
Development of a long-acting drug-delivery system of the peptidic somatostatin agonist octreotide; and,
Design and application of a biotinylated probe of calenduloside E.
| Unit Size | 100 mg, 1000 mg |
|---|---|
| Molecular Weight | 218.25; single compound |
| Chemical formula | C₈H₁₈N₄O₃ |
| CAS | 134179-38-7 |
| Purity | > 98% |
| Spacers | dPEG® Spacer is 14 atoms and 15.4 Å |
| Shipping | Ambient |
| Typical solubility properties (for additional information contact Customer Support) | Methylene chloride, Acetonitrile, DMAC, or DMSO. |
| Storage and handling | -20°C; Always let come to room temperature before opening; be careful to limit exposure to moisture and restore under an inert atmosphere; stock solutions can be prepared with dry solvent and kept for several days (freeze when not in use). dPEG® pegylation compounds are generally hygroscopic and should be treated as such. This will be less noticeable with liquids, but the solids will become tacky and difficult to manipulate, if care is not taken to minimize air exposure. |
Greg T. Hermanson, Bioconjugate Techniques, 3rd Edition, Elsevier, Waltham, MA 02451, 2013, ISBN 978-0-12-382239-0; See Chapter 18, Discrete PEG Reagents, pp. 787-821, for a full overview of the dPEG® products.
Detection of SK2 Channels on Hippocampal Neurons. Jamie L. Maciaszek University of Connecticut (2012) DigitalCommons@Uconn Master’s Theses Paper 237 April 20, 2012. http://digitalcommons.uconn.edu/gs_theses/237.
Epinephrine Modulates BCAM/Lu and ICAM-4 Expression on the Sickle Cell Trait Red Blood Cell Membrane. Jamie L. Maciaszek, Biree Andemariam, Greg Huber, and George Lykotrafitis. Biophysical Journal. 2012, 102 pp 1137–1143. January 27, 2012. DOI: 10.1016/j.bpj.2012.01.050.
Subcutaneously Administered Self-Cleaving Hydrogel-Octreotide Conjugates Provide Very Long-Acting Octreotide. Eric L. Schneider, Jeff Henise, Ralph Reid, Gary W. Ashley, and Daniel V. Santi. Bioconjugate Chemistry. 2016, June 2, 2016. DOI: 10.1021/acs.bioconjchem.6b00188.
The proteomic profiling of calenduloside E targets in HUVEC: design, synthesis and application of biotinylated probe BCEA. Yu Tian, Shan Wang, Hai Shang, Min Wang, Guibo Sun, Xudong Xu, and Xiaobo Sun. Royal Society of Chemistry. 2017, 7, pp 6259-6265. January 18, 2017. DOI: 10.1039/c6ra25572h.
Applicable patents and legal notices are available at legal notices.
Stay in the Loop. Join Our Online Community
Products
Ordering
About Us
Application
Resources

©Vector Laboratories, Inc. 2025 All Rights Reserved.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Privacy Statement
How do I Request a Quote?
To request a quote for products: