Maleimide Reaction Chemistry
The simple, rapid reaction between a thiol and a maleimide to generate a thiosuccinimide product (Figure 1) is currently one of the most popular methods for site-selective modification of cysteine residues in bioconjugation technology.[1], [2] Although the thioether bond formed between a sulfhydryl and a maleimide group is slowly reversible under certain conditions (see discussion below), the maleimide moiety itself is relatively stable to degradation[3], allowing our customers to work easily with the product to achieve their desired conjugates.
Figure 1

Figure 1: The reaction between a free thiol and a maleimide produces a thiosuccinimide product. The process is a type of “click chemistry” reaction.
Is the Thiol-Maleimide Reaction a Click Chemistry Reaction?
The short answer to this question is, “Yes, the thiol-maleimide reaction is a type of click chemistry reaction”. Specifically, the reaction between a free thiol and a maleimido group is an addition reaction known as the thiol-Michael addition [4] and also as the thiol-maleimide reaction [5]. Many scientists consider this reaction to be a type of “click chemistry” reaction because it meets most of the criteria for “click chemistry” as defined by Kolb, Finn, and Sharpless [4], [6] (Figure 2).
What makes a reaction Click Chemistry?
According to Kolb, Finn, and Sharpless, for a process to be click chemistry, the reaction must
be modular
be wide in scope
give very high chemical yields
generate only inoffensive byproducts that can be removed by non-chromatographic methods
be stereospecific (but not necessarily enantioselective).
The process should have the following characteristics:
has simple reaction conditions
use readily available starting materials and reagents
use no solvent, a benign solvent such as water, or an easily removed solvent
provide simple, non-chromatographic product isolation (e.g., crystallization or distillation).
In addition, the reaction product must be stable under physiological conditions. Furthermore, the reaction must have a large thermodynamic driving force (>20 kJ/mole) that favors a single reaction product. Such reactions are deemed to be “spring-loaded.”
Figure 2: What makes a reaction “click chemistry”? Adapted from Reference 6, vide infra.
What is the Mechanism of the Thiol-Maleimide Reaction?
Figure 3 shows the general mechanism of the thiol-maleimide reaction. Figure 1 of reference 5 below contains a more detailed reaction scheme. The reason for the high reactivity of the olefin is due primarily to (a) the ring strain arising from the bond angle distortion and (b) the positioning of the carbonyl groups in the cis-conformation. Indeed, in highly polar solvents such as water, dimethyl sulfoxide (DMSO), N,N’-dimethylformamide (DMF), or N,N’-dimethylacetamide (DMAC), the thiol-maleimide reaction proceeds without a catalyst, because the polar solvent forms the thiolate ion, which is the active species for the reaction.[4]
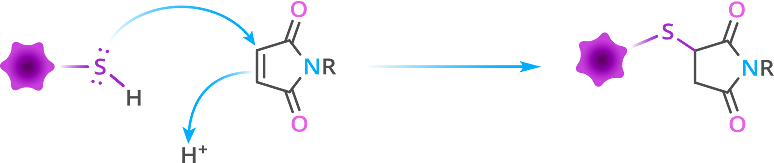
Figure 3: Simplified general mechanism of the thiol-maleimide reaction, which is a specific type of Michael addition reaction.
From pH 6.5 to pH 7.5, the thiol-maleimide reaction is chemoselective for thiols. At pH 7.0, the reaction rate of maleimide with thiols is about 1,000 times faster than the reaction rate of maleimide with amines. However, above pH 7.5, free primary amines react competitively with thiols at the maleimide C=C bond (Figure 3).1
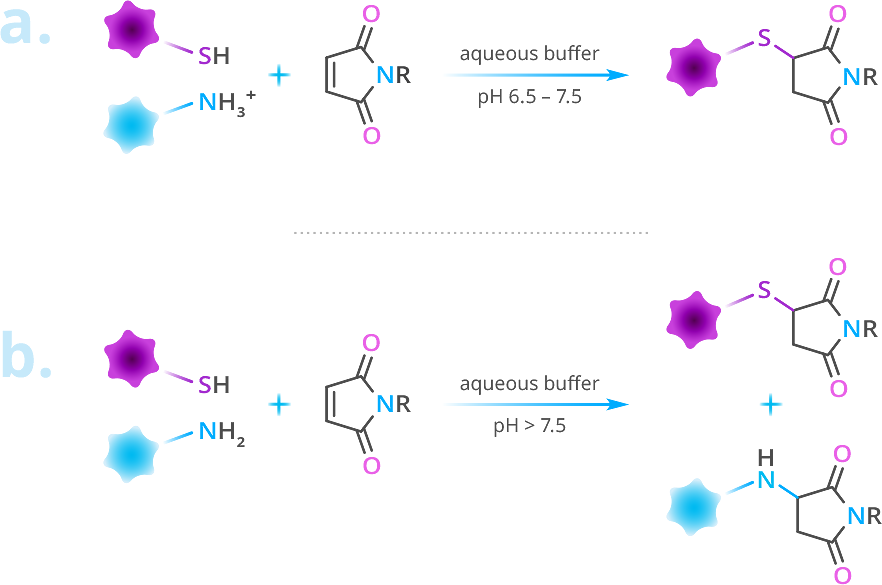
Figure 4: The thiol-maleimide reaction. (a) The reaction is chemoselective for thiols from pH 6.5 – 7.5. (b) Above pH 7.5, thiol chemoselectivity is lost, and the maleimide moiety begins reacting with free amines (e.g., lysine).
Is the Thiosuccinimide Conjugate Stable?
In aqueous solution, the maleimide ring can be opened by hydrolysis (Figure 5). This susceptibility to hydrolysis increases with increasing pH. Importantly, if the ring-opening reaction occurs before thiolation, the resultant maleic amide is unreactive to thiols. On the other hand, if thiolation has occurred, the ring-opened succinamic acid thioether is stable. Because of the propensity for ring-opening hydrolysis and inactivation, we at Vector Laboratories do not recommend aqueous storage for products containing a maleimide, which is intended for future reactions. If solution storage is required, use a dry, water-miscible, biocompatible solvent such as DMSO, DMF, or DMAC (see below for more information).
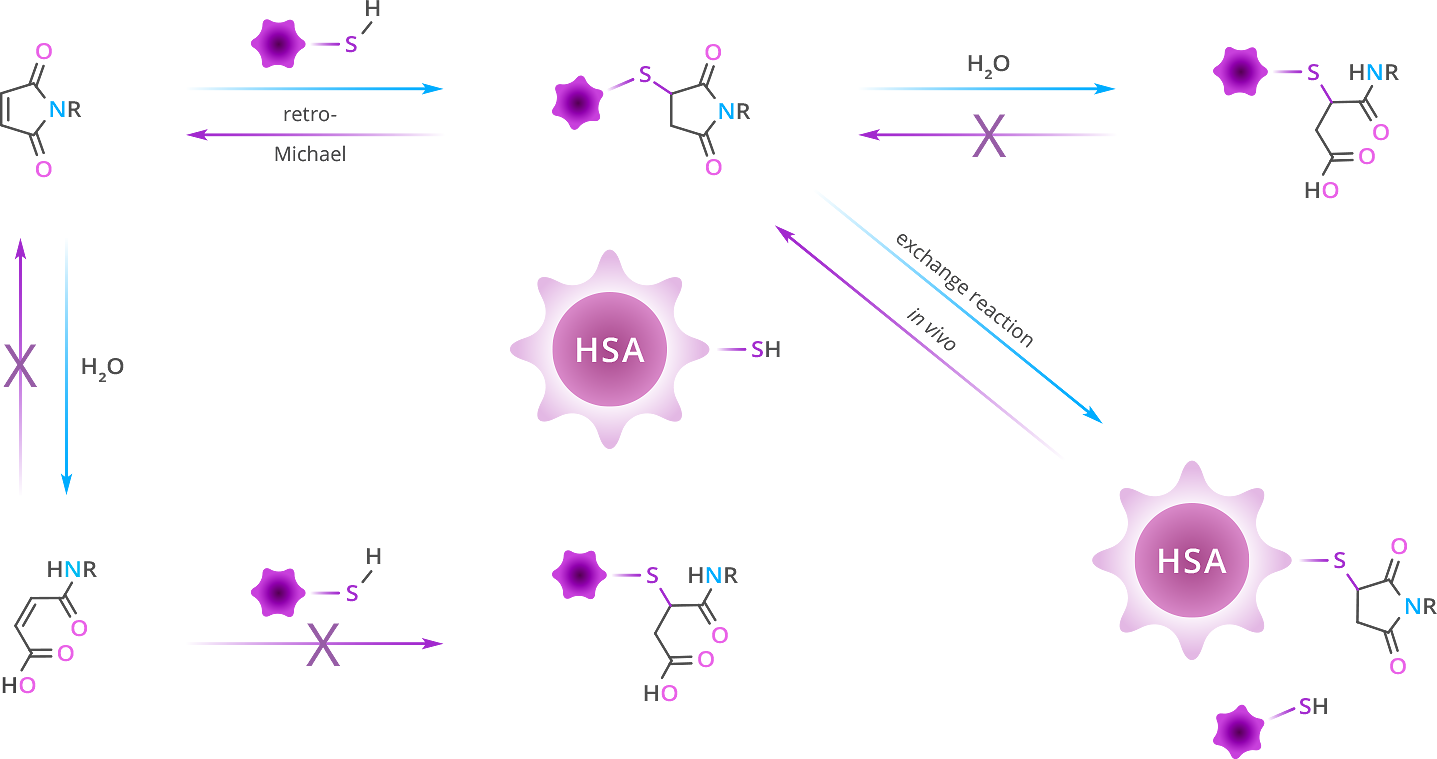
Figure 5: The reactions of thiol and maleimide. See the text for a complete description of these reactions.
As shown in the top left portion of Figure 5, the thiosuccinimide product can undergo a retro-Michael reaction to regenerate the maleimide. The reformed maleimide is then free to react with the same or a different thiol. In vivo, this retro-Michael reaction can lead to so-called “payload migration”, as shown by the exchange reaction in Figure 5 on the lower right-hand side.[7] Research into payload migration and the stability of thiosuccinimide groups on antibody-drug conjugates (ADCs) has shown that the stability of the thiosuccinimide ring depends on the site of conjugation on the antibody[8], [9]. The reformed maleimide can then react with other serum proteins such as serum albumin, causing the payload to exert off-target effects. The thiosuccinimide ring should be hydrolyzed after the conjugation reaction and workup of the conjugated product to avoid payload migration when using the thiol-maleimide conjugation.[10], [11], [12], [13], [14]
In contrast, the thiol-bromoacetamide reaction leads to a stable thioether product that is not as susceptible to the reverse reaction as the thiol-maleimide conjugation reaction. However, the thiol-bromoacetamide reaction is slower and requires a higher pH to proceed. Furthermore, it is not as chemoselective as the thiol-maleimide reaction.
Working with Maleimide-Containing Products in Thiol-Maleimide Reactions
Vector Laboratories‘ maleimide-containing dPEG® products are easy to use effectively following our recommended instructions on the product information sheets that are included with every order. The following are some recommended best practices for working with our maleimide-containing dPEG® products.
- Products should be stored at the recommended temperature except when in use. The recommended storage temperature for almost all products that contain maleimide is -20 °C.
- When a product containing maleimide is removed from storage, it should be allowed to equilibrate fully to ambient temperature before opening the bottle or vial in which the product is stored.
- Aqueous solutions of maleimide-containing products should be made immediately before use. If the maleimide functional group is to be reacted, the pH should be 6.5 – 7.5, and preferably as low as possible within that range.
- Aqueous buffers and organic solutions of maleimide-containing products should be free of primary and secondary amines and free of thiols. If a base needs to be used in a reaction with a maleimide-containing product, we recommend a highly hindered organic base such as 2,6-lutidine (CAS number 108-48-5; EC number 203-587-3).
- Do not store maleimide-containing products in aqueous solutions due to the risk of hydrolysis. Instead, use a dry, biocompatible, water-miscible solvent (g., DMSO, DMF, or DMAC) for the long-term storage of these compounds. These solvents can be dried suitably over 3 Å molecular sieves (8×12 mesh recommended) for 24 – 48 hours at 20 –25 °C. Solubilized maleimide-containing products should be stored at –20 °C. Vector Laboratories does not have information on the stability of maleimide-containing products stored in solution because we explicitly do not recommend such storage for these products.
- When conjugating a maleimide containing product to a protein, if a stock solution of the maleimide-containing product in an organic solvent (vide supra) is added to the reaction mixture, no more than 10% of the final reaction volume should be the organic solvent, while the rest of the volume should be water or aqueous buffer (for example, PBS). Some sensitive proteins may require that the amount of organic solvent be much less than 10% of the final reaction volume.
References
- Hermanson, G. T. Chapter 3, The Reactions of Bioconjugation. Bioconjugate Techniques, 3rd edition. Academic Press: New York, 2013, 229-258, specifically page 241, discussing maleimide reactions.
- Ravasco, J. M. J. M.; Faustino, H.; Trindade, A.; Gois, P. M. P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chemistry – A European Journal 2019, 25(1), 43–59. [Chemistry Europe]
- Hermanson, G. T. Chapter 2, Functional Targets for Bioconjugation. Bioconjugate Techniques, 3rd edition. Academic Press: New York, 2013, 127-228, specifically page 148, discussing maleimide reactions.
- Nair, D. P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C. R.; Bowman, C. N. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater. 2014, 26(1), 724–744. [ACS Publications].
- Northrop, B. H.; Frayne, S. H.; Choudhary, U. Thiol–Maleimide “Click” Chemistry: Evaluating the Influence of Solvent, Initiator, and Thiol on the Reaction Mechanism, Kinetics, and Selectivity. Polym. Chem. 2015, 6(18), 3415–3430. [Publishing].
- Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40(11), 2004–2021. [Wiley].
- Dong, L.; Li, C.; Locuson, C.; Chen, S.; Qian, M. G. A Two-Step Immunocapture LC/MS/MS Assay for Plasma Stability and Payload Migration Assessment of Cysteine–Maleimide-Based Antibody Drug Conjugates. Anal. Chem. 2018, 90(10), 5989–5994. [ACS Publications].
- Shen, B.-Q.; Xu, K.; Liu, L.; Raab, H.; Bhakta, S.; Kenrick, M.; Parsons-Reponte, K. L.; Tien, J.; Yu, S.-F.; Mai, E.; et al. Conjugation Site Modulates the in Vivo Stability and Therapeutic Activity of Antibody-Drug Conjugates. Nature Biotechnology 2012, 30 (2), 184–189. [Nature Biotechnology].
- Zheng, K.; Chen, Y.; Wang, J.; Zheng, L.; Hutchinson, M.; Persson, J.; Ji, J. Characterization of Ring-Opening Reaction of Succinimide Linkers in ADCs. Journal of Pharmaceutical Sciences 2019, 108(1), 133–141. [Journal of Pharmaceutical Sciences].
- Tumey, L. N.; Charati, M.; He, T.; Sousa, E.; Ma, D.; Han, X.; Clark, T.; Casavant, J.; Loganzo, F.; Barletta, F.; et al. Mild Method for Succinimide Hydrolysis on ADCs: Impact on ADC Potency, Stability, Exposure, and Efficacy. Bioconjugate Chem. 2014, 25(10), 1871–1880. [ACS Publications].
- Fontaine, S. D.; Reid, R.; Robinson, L.; Ashley, G. W.; Santi, D. V. Long-Term Stabilization of Maleimide–Thiol Conjugates. Bioconjugate Chem. 2015, 26(1), 145–152. [ACS Publications].
- Christie, R. J.; Fleming, R.; Bezabeh, B.; Woods, R.; Mao, S.; Harper, J.; Joseph, A.; Wang, Q.; Xu, Z.-Q.; Wu, H.; et al. Stabilization of Cysteine-Linked Antibody Drug Conjugates with N-Aryl Maleimides. Journal of Controlled Release 2015, 220, 660–670. [ScienceDirect].
- Ponte, J. F.; Sun, X.; Yoder, N. C.; Fishkin, N.; Laleau, R.; Coccia, J.; Lanieri, L.; Bogalhas, M.; Wang, L.; Wilhelm, S.; et al. Understanding How the Stability of the Thiol-Maleimide Linkage Impacts the Pharmacokinetics of Lysine-Linked Antibody–Maytansinoid Conjugates. Bioconjugate Chem. 2016, 27 (7), 1588–1598. [ACS Publications].
- Szijj, P. A.; Bahou, C.; Chudasama, V. Minireview: Addressing the Retro-Michael Instability of Maleimide Bioconjugates. Drug Discovery Today: Technologies 2018, 30, 27–34. [ScienceDirect].

