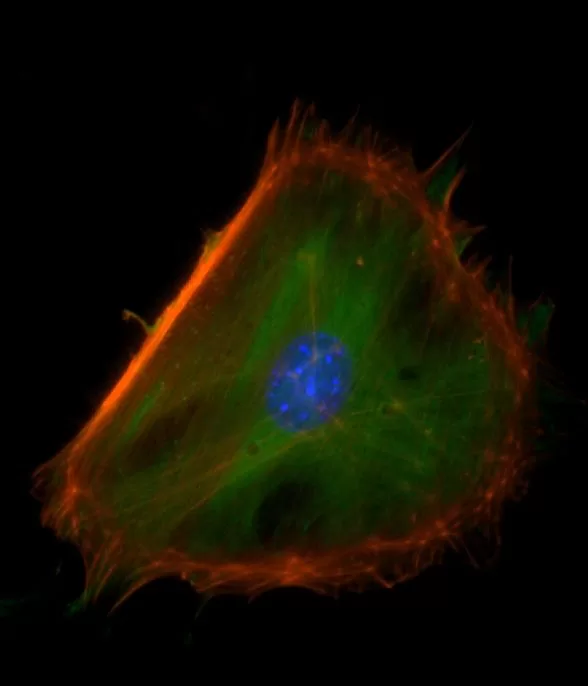
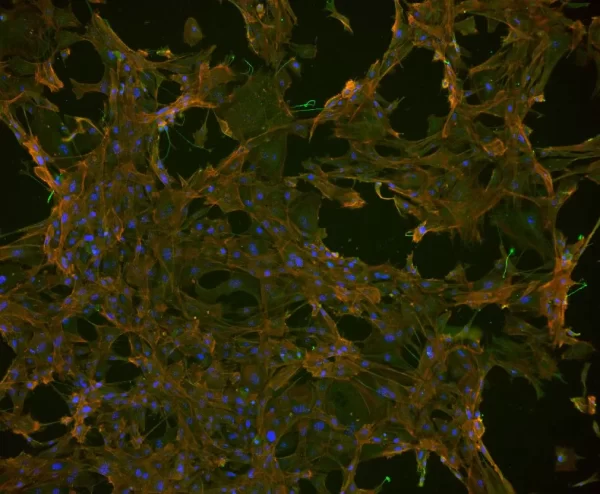
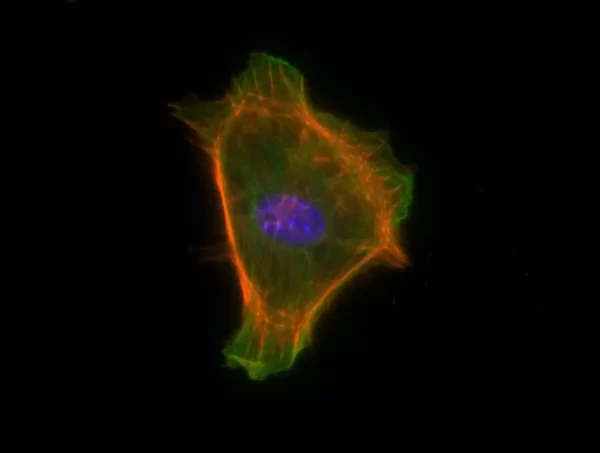
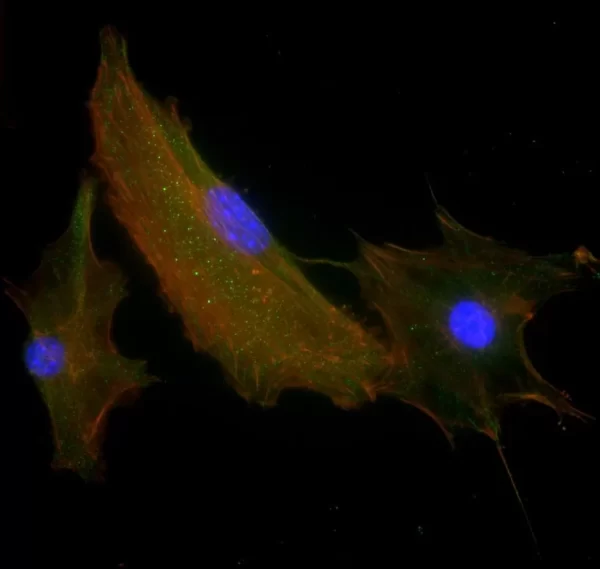
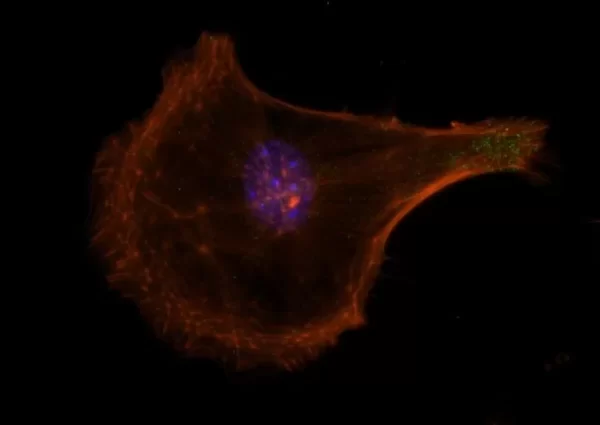
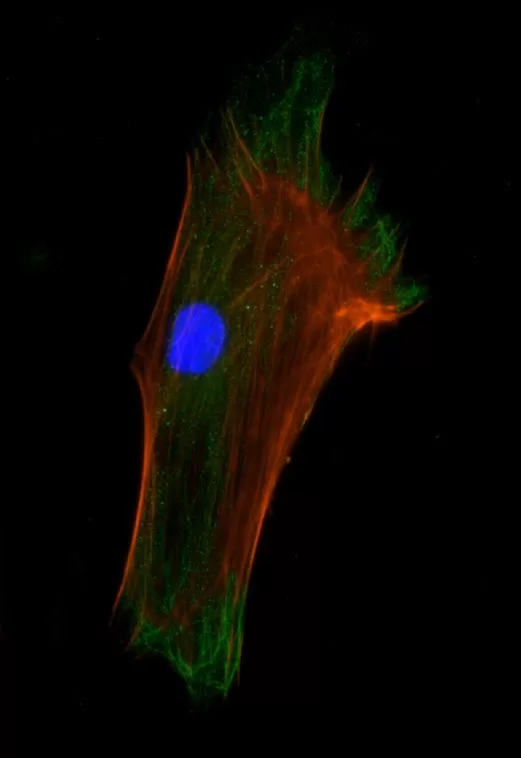
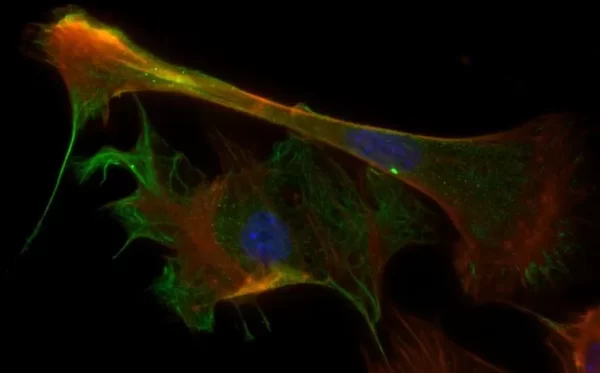
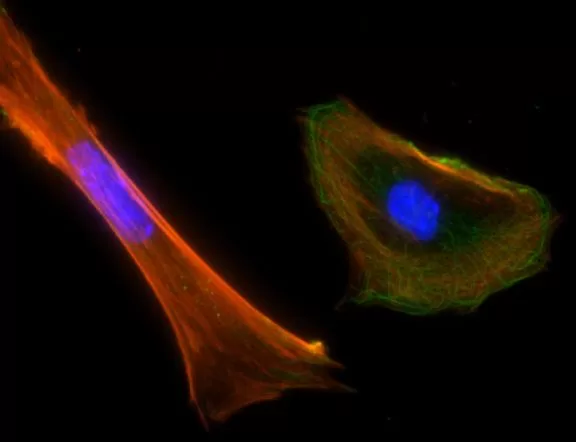
Description
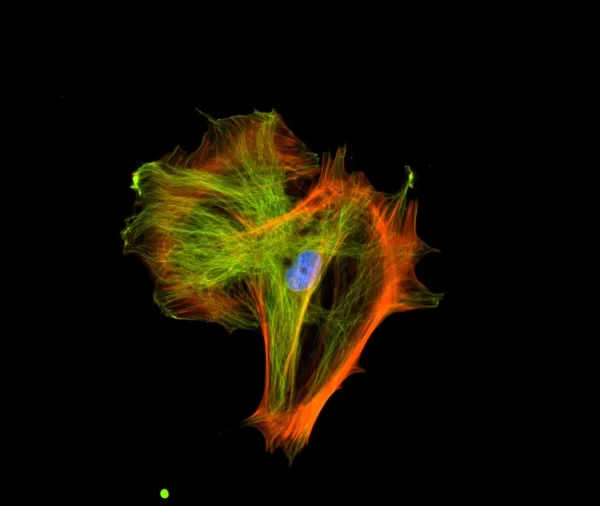
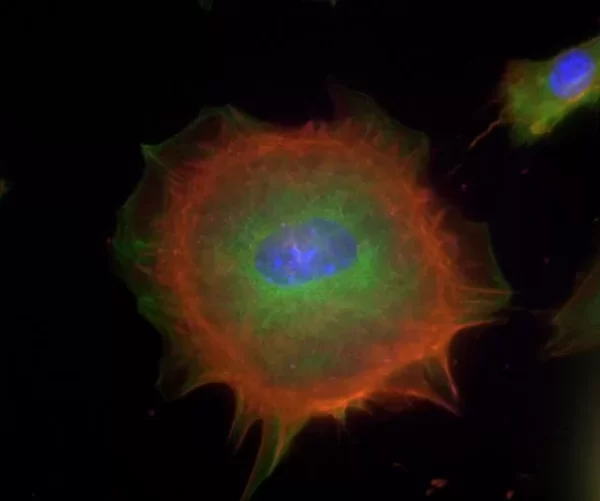
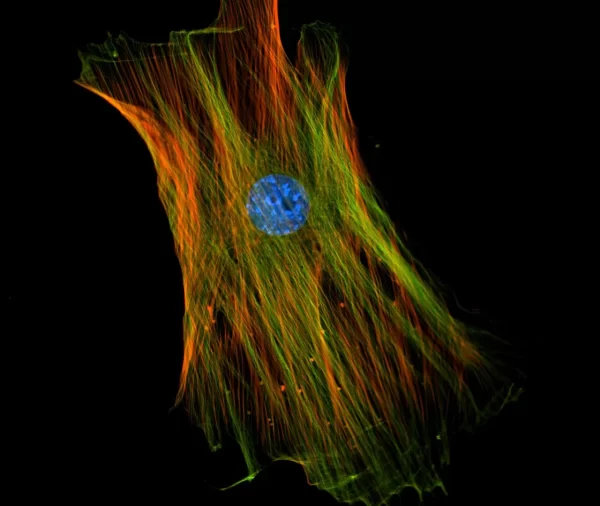
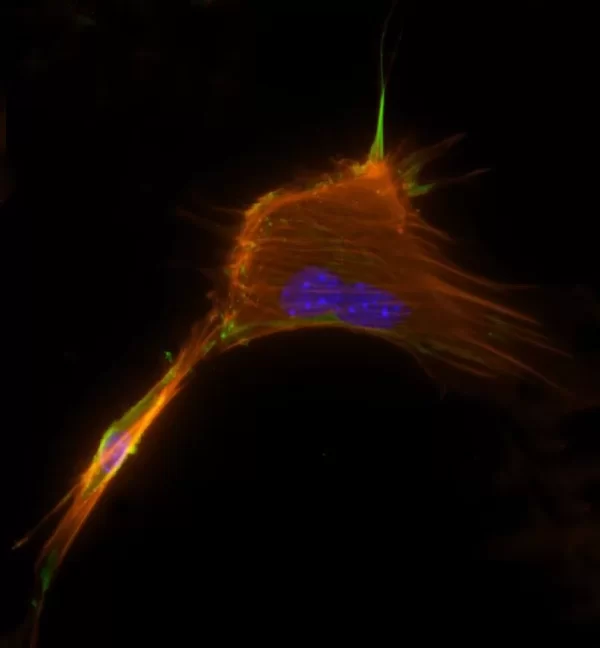
The VECTASHIELD HardSet Antifade Mounting Medium with TRITC-Phalloidin prevents rapid photobleaching of fluorescent proteins and fluorescent dyes. Fluorescent derivatives of phalloidin are used to stain actin filaments. TRITC (tetramethylrhodamine) is excited at 544 nm and emits at 572 nm, producing an orange-red fluorescence. VECTASHIELD HardSet Mounting Media hardens after coverslipping. After approximately 15 minutes at room temperature, the coverslip will become immobilized, and optimal antifade ability and refractive index will be achieved.
Features:
- Ideal refractive index (1.46)
- Ready-to-use – from the refrigerator to the benchtop
- No warming necessary
- Can be stored without sealing for long term analysis
- Hardening formulation
Specifications
| Unit Size | 10 ml |
|---|
| Applications | Immunofluorescence, In situ hybridization, Cellular Imaging |
|---|
| Mounting | Aqueous (Hardening) |
|---|
| Antifade | Yes |
|---|
| Counterstain | Phalloidin Rhodamine (red fluorescence) |
|---|
| Cellular Stain | Cytoskeleton |
|---|
Product FAQs
All of the VECTASHIELD formats we currently offer which includes regular non-setting VECTASHIELD, PLUS, HardSet and Vibrance formulations each contain a percentage of glycerol.
No form of tissue dehydration (e.g. air drying or ethanol exposure) is required nor recommended when applying VECTASHIELD. From our experience, the most optimal antifade actions of VECTASHIELD are obtained when the preparation is removed from the final buffer/water rinse, kept slightly wet/moist and then coverslipped with a small volume (25-50 uL) of VECTASHIELD.
That depends upon how long you wish to retain the slides and which VECTASHIELD formulation you are using. If you are using one of our non-setting VECTASHIELD products such as H-1000/H-1200 or H-1900/H-2000, then we suggest sealing the coverslip with plastic sealant or nail polish if you intend to keep the slides beyond a day or so. If you are using one of our setting/curing formulations such as VECTASHIELD HardSet or Vibrance, then in most cases when using thin cut (<10 um) tissue sections or cell monolayers, no sealing of the coverslip is requuired.
Technical Information
VECTASHIELD Mounting Media are compatible with a wide array of fluorochromes, enzymatic substrates, and fluorescent proteins. Please consult the compatibility table (in the “Documents” section) to determine if VECTASHIELD will be compatible in your system.
All formulations of VECTASHIELD Antifade Mounting Media are available with or without the counterstain DAPI (4′, 6-diamidino-2-phenylindole). The DAPI concentration can be modified by mixing with the corresponding VECTASHIELD Mounting Medium without DAPI. DAPI produces a blue fluorescence when bound to DNA with excitation at about 360 nm and emission at 460 nm.
R.J. Florijn, et. al. Cytometry, 19 (1995) 177-182.
The refractive index for VECTASHIELD Mounting Medium is 1.45.
VECTASHIELD Mounting Medium in Super Resolution Microscopy
The optimal medium for super-resolution imaging methods maximizes the lifetime and photoswitching characteristics of fluorophores. VECTASHIELD Mounting Medium has been shown to be compatible with a number of fluorophores used in super resolution methods including STORM, STED, and 3D-SIM imaging, such as Cy5 or Alexa Fluor 647, resulting in greater convenience and reproducibility of the method. (Olivier, N. et al., 2013, Biomedical Optics Express, Vol. 4 No. 6, pp. 885-899; Glushonkov, O. et al., 2018, Scientific Reports, Vol. 8, 8749 (2018) ).
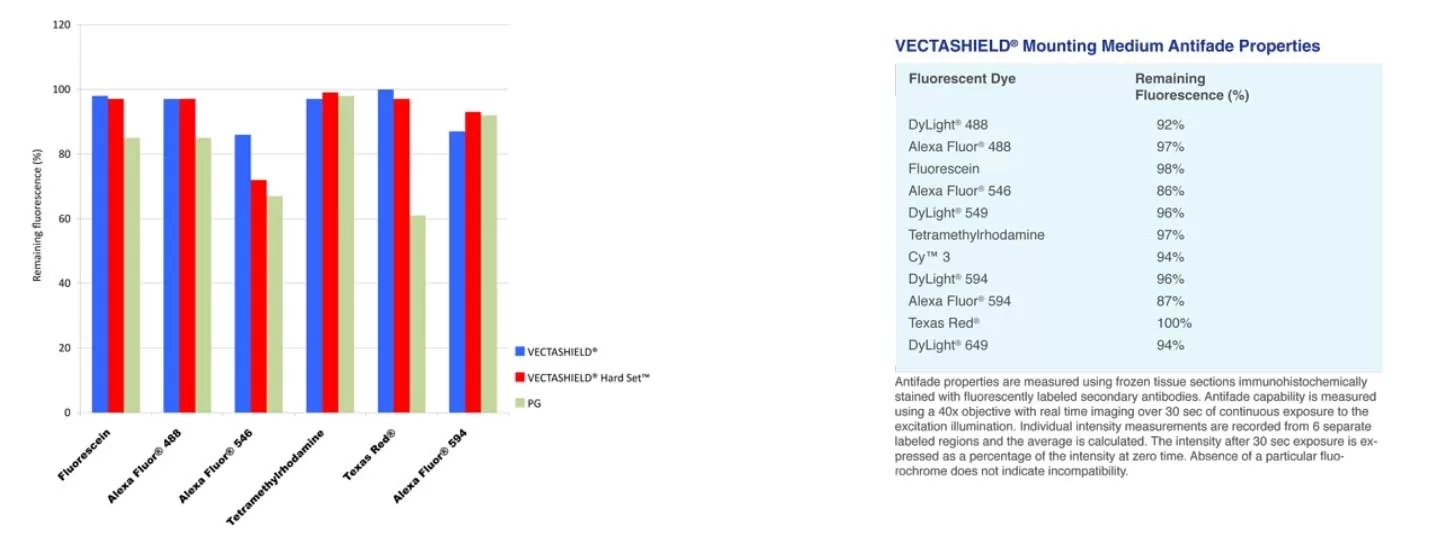
VECTASHIELD Mounting Medium Antifade Comparison
Other manufacturers measure the antifade properties of their mountants using labeled microspheres or arrayed spots. Vector Labs prefers to measure antifade properties of VECTASHIELD mountants using frozen tissue sections immunohistochemically stained with fluorescently labeled secondary antibodies. Antifade capability is measured using a 40x objective with real time imaging over 30 seconds of continuous exposure to the excitation illumination. Individual intensity measurements are recorded from 6 separate labeled regions and the average is calculated. The intensity after 30 second exposure is expressed as a percentage of the intensity at zero time. The values for PG are taken from the manufacturer s published results.
Check out the below video for coverslipping tips for immunofluorescence slide mounting