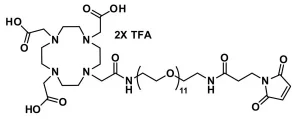
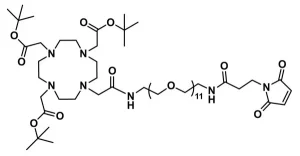
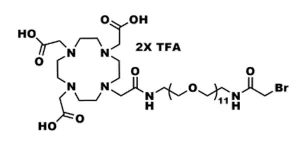
DOTA-tris(acid)-amido-dPEG®3-bromoacetamide, product number QBD-11150, combines the macrocycle 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) through a single molecular weight, discrete polyethylene glycol (dPEG®) linker to a thiol-reactive bromoacetyl moiety that couples to the dPEG® linker via an amide bond. The linker contains 14 atoms and is 15.6 Å long from the terminal amide adjacent to the DOTA moiety to the methylene moiety adjacent to the bromine atom. This product is useful in radioimaging and radiotherapeutic applications.
DOTA is a highly popular bifunctional chelator used in a variety of diagnostic and therapeutic applications for the delivery of radionuclides, particularly lanthanide radionuclides and certain rare earth radioisotopes such as yttrium. For treatments using trivalent lanthanide radioisotopes, DOTA is the preferred ligand because it forms thermodynamically stable, kinetically inert complexes.
The bromoacetyl moiety (present as the bromoacetamide in QBD-11150) reacts chemoselectively, but not chemospecifically, with free thiol groups to form stable thioether bonds. The rate and selectivity of bromoacetyl for free thiols depend upon the pH of the reaction and the relative availability (compared to thiols) of other groups with which the bromoacetyl moiety can react (for example, surface-accessible primary amines).
The short, single molecular weight dPEG® linker between the DOTA and the bromoacetyl groups serves many purposes. It provides flexibility to the entire molecule. Because each ethylene glycol unit in the dPEG® chain hydrogen bonds up to three molecules of water, the molecule gains water solubility, and in aqueous environments such as blood, it increases the hydrodynamic volume of the molecule and of anything to which the molecule is conjugated. With larger hydrodynamic volume, conjugates are less susceptible to renal excretion, which means that lower doses of the diagnostic or therapeutic agent are needed for the conjugate to affect its purpose. Moreover, dPEG® is non-immunogenic, and its large hydrodynamic volume helps reduce the immunogenicity of molecules to which it is conjugated.
| Unit Size | 50 mg, 100 mg, 500 mg |
|---|---|
| Molecular Weight | 927.636; single compound |
| Chemical formula | C₃₀H₄₉BrF₆N₆O₁₅ |
| CAS | N/A |
| Purity | > 95% |
| Spacers | dPEG® Spacer is 14 atoms and 15.6 Å |
| Shipping | Ambient |
| Typical solubility properties (for additional information contact Customer Support) | Methylene Chloride, Chloroform, Acetonitrile, Methanol, DMSO, or water. |
| Storage and handling | -20°C; Always let come to room temperature before opening; be careful to limit exposure to moisture and restore under an inert atmosphere; stock solutions can be prepared with dry solvent and kept for several days (freeze when not in use). dPEG® pegylation compounds are generally hygroscopic and should be treated as such. This will be less noticeable with liquids, but the solids will become tacky and difficult to manipulate, if care is not taken to minimize air exposure. |
Greg T. Hermanson, Bioconjugate Techniques, 2nd Edition, Elsevier Inc., Burlington, MA 01803, April, 2008 (ISBN-13: 978-0-12-370501-3; ISBN-10: 0-12-370501-0). Specifically see pp. 726-729 in his Chapter 18 on discrete PEG compounds for pegylation applications.
Greg T. Hermanson, Bioconjugate Techniques, 3rd Edition, Elsevier, Waltham, MA 02451, 2013, ISBN 978-0-12-382239-0; See chapter 18, Discrete PEG Reagents, pp.787-821, for a full overview of the dPEG® products.
Applicable patents and legal notices are available at legal notices.
Stay in the Loop. Join Our Online Community
Products
Ordering
About Us
Application
Resources

©Vector Laboratories, Inc. 2025 All Rights Reserved.
To provide the best experiences, we use technologies like cookies to store and/or access device information. Consenting to these technologies will allow us to process data such as browsing behavior or unique IDs on this site. Not consenting or withdrawing consent, may adversely affect certain features and functions. Privacy Statement
How do I Request a Quote?
To request a quote for products: