Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
The S-4FB heterobifunctional crosslinker is fundamental to the SoluLINK bioconjugation technology. S-4FB reacts with primary amines on proteins (through lysine amino acids) or amino-modified oligonucleotides or surfaces, introducing a 4FB linker that forms stable covalent conjugates with biomolecules possessing HyNic linkers.
| Label/Modifier Type | 4FB |
|---|---|
| Reactivity | HyNic |
| Recommended Storage | Desiccated: -15° to -25°C |
| Applications | Antibody Labeling, Antisense/RNAi, Aptamers |
| Other Name(s) | SoluLINK Bioconjugation |
This core technology is based on the formation of a covalent bond formed from an aromatic hydrazine and an aromatic aldehyde. S- HyNic 1 (succinimidyl 6-hydrazinonicotinate acetone hydrazone, SANH) is used to incorporate aromatic hydrazine linkers on biomolecules. S-HyNic is an amino-reactive reagent that directly converts amino groups on biomolecules and surfaces to HyNic groups. S-4FB 2 (succinimidyl 4-formylbenzoate, SFB) is used to convert amino groups to aromatic aldehydes (4-formylbenzamide (4FB) groups). Addition of a HyNic-modified biomolecule to a 4FB-modified biomolecule or surface directly leads to the formation of the conjugate (Figure 1). The conjugate bond is stable to 92°C and pH 2.0- 10.0. The recommended pH for antibody conjugation is 6.0. Unlike thiol-based conjugation protocols where reducing reagents are required that that can compromise the activity of proteins by cleaving disulfide bonds, the HyNic-4FB conjugation couple leaves disulfide bonds intact. No oxidants, reductants or metals are required in the preparation of conjugate.
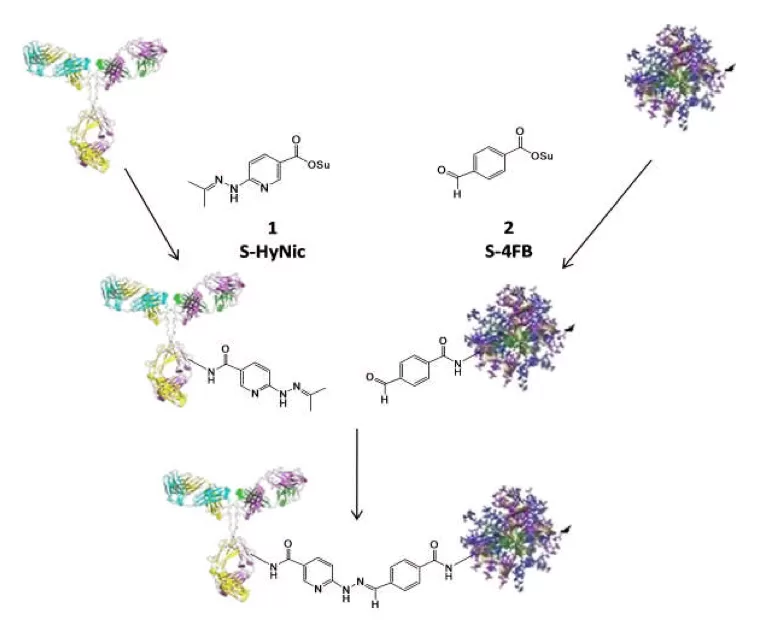
Figure 1: Schematic representation of the SoluLINK bioconjugation chemistry where an antibody is modified with S-HyNic to incorporate HyNic groups and a second protein is modified with S-4FB to incorporate 4FB groups. Conjugate is formed directly by simply mixing the HyNic-modified antibody with the 4FB-modified proteins.
Further enhancing the many advantages of the HyNic/4FB conjugation couple is the discovery by Dirksen et al. that showed that aniline catalyzes the formation of this Schiff’s base. This is especially effective for large biomolecule conjugations. In the case of antibody-protein conjugations the addition of 10 mM TurboLINK Catalyst Buffer (aniline) to the reaction mixture converts >95% of the antibody to conjugate in ~2 hours using 1-2 mole equivalents of second protein.
The HyNic-4FB conjugation couple is chromophoric- the conjugate bond absorbs at 354 nm and has a molar extinction coefficient of 29,000 L/(mol*cm). This allows (1) real time spectrophotometric monitoring of a conjugate reaction, (2) ability to visualize the conjugate during chromatographic purification using a UV or photodiode array detector and (3) quantification of conjugation.
SoluLINK® Bioconjugation For Research Use Only. Not for use in diagnostic procedures. For additional licensing restrictions, please see the license agreement at vectorlabs.com/solulink-research-license.
Products are for research use only, not for use in diagnostic or therapeutic procedures or for use in humans. Products are not for resale without express written permission of Seller. No license under any patent or other intellectual property right of Seller or its licensors is granted or implied by the purchase unless otherwise provided in writing.
Applicable patents and legal notices are available at legal notices.



Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.
How do I Request a Quote?
To request a quote for products: