
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
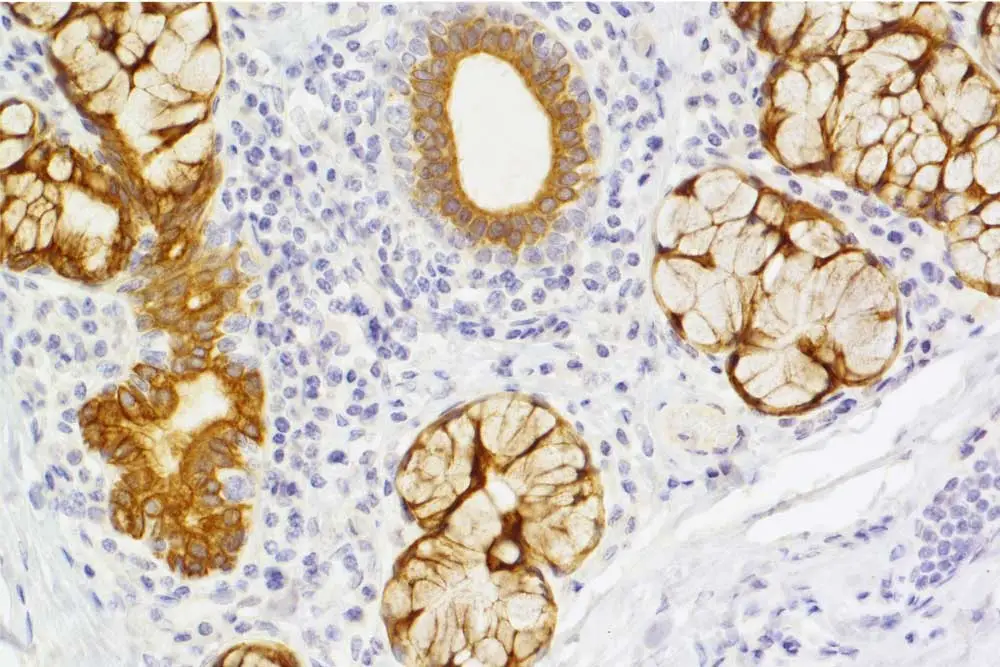
For researchers wishing to know not only “if” but “where” a protein or molecule of interest is expressed within a tissue, immunohistochemistry (IHC) is an invaluable and accessible technique. Although less quantitative than immunoassays like Western blotting and ELISA, the appeal of IHC is that it preserves the tissue’s spatial information, allowing researchers to visualize the pattern of expression of a protein in relation to other molecules and structures that surround it.
Moving beyond studying basic biology, IHC is an important tool in drug development and disease research, used to detect changes in disease marker expression post-treatment, as well as in therapeutic antibody development. In these studies, human or humanized antibodies are often used to treat different cancers and autoimmune conditions. IHC helps scientists confirm that a therapeutic antibody will bind only to an intended target, not other non-target epitopes.
The US Food and Drug Administration (FDA) recommends that antibody-drug candidates undergo tissue-cross reactivity (TCR) tests against 42 human tissue to confirm on-target binding and assess any off-target binding that could cause adverse effects (1,2). Chromogenic IHC is currently a preferred technology for TCR testing.
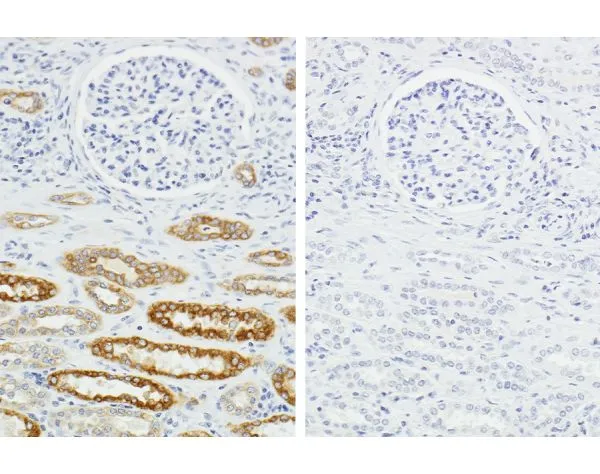
Most therapeutic antibodies are either fully human or humanized making standard IHC methods impractical due to high concentrations of endogenous human immunoglobulins. Indirect detection by a secondary antibody directed against anti-human IgG cannot distinguish between the therapeutic antibody and endogenous IgG, resulting in background staining. Although background can be reduced by lowering primary antibody concentration or shortening incubation times, high assay sensitivity is essential to avoid missing real off-target effects.
There are two main methods currently used to get around this issue: indirect detection using a molecular tag (hapten)—usually biotin or fluorescein isothiocyanate (FITC)—or precomplexing the human antibody with anti-human IgG secondary antibodies. Indirect detection of the hapten label requires covalent modification of the therapeutic antibody, which can affect the target binding characteristics. The second method involves pre-complexing the human antibody with anti-human IgG secondary antibody, and then using human IgG to block unbound secondary antibody. Optimizing the ratios of these antibodies is complex and background can be tricky to eliminate.
The Vector Laboratories H.O.H.™ (Human on Human) Immunodetection Kit offers another approach to the screening of humanized antibodies in human tissue. It has been shown to achieve high sensitivity of target antigens while excluding background from endogenous antibodies. The H.O.H. Kit uses a straightforward two-step primary antibody preparation followed by standard IHC assay detection that can be used to detect human or humanized antibodies on frozen or paraffin-embedded human tissue sections. It greatly reduces assay time by eliminating the need to conjugate a primary antibody, achieving high sensitivity of target antigens while eliminating background from endogenous IgG.
The H.O.H. Kit is a comprehensive HRP (peroxidase) kit complete with volume matched reagents and ImmPACT DAB EqV substrate that can be used with multiple human isotypes and antibody configurations, including bivalent antibodies without Fc regions. An easy-to-follow protocol (no tedious calculations required) leads to clear, specific, background-free staining all in about 90 minutes following initial antibody preparation (~1 hour incubation).
You can read more about Vector Laboratories’ H.O.H. Kit and how it compares to other methods in this white paper. And don’t hesitate to reach out with questions specific to your research goals — connect with an expert to learn more.
References





Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.