
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.

Heart diseases comprise many different conditions, and together, they are responsible for about 20% of all deaths in the United States (1). Coronary heart diseases—blockage or narrowing of the heart’s main blood vessels—are the most common type of heart disease and can lead to myocardial infarction (MI), a serious and life-threatening condition (1). MI happens when blood flow to the heart completely stops. As a result, lack of oxygen and nutrients results causes damage to the heart muscle and contribute to heart failure (1).
Current treatments for MI focus on preventing reoccurrence. Patients often receive medications to prevent blood clots, improve blood flow, slow heart rate, and reduce blood pressure (2). Clinical management of MI still lacks interventions focused on counteracting pathophysiological changes and preventing damage to the heart muscle. The development of such treatments can have a major impact on public health, as it has the potential to improve health outcomes in patients who have had MI.
Researchers from the Oregon Health and Science University, Sepe et al., characterized pathophysiological changes in the heart in response to novel therapeutics that disrupt protein tyrosine phosphatase-s signaling (3). They observed that the treatment restores sympathetic innervation and shifts the immune response after MI to promote tissue repair. As a result, heart function improved and susceptibility to arrhythmia decreased (3).
Keep reading to learn more details about the research study, and check out the blog for other publication highlights.
After MI, 2 waves of immunological response recruit and activate innate and adaptative immune cells to clear cellular debris and remodel the infarcted heart (4). A pro-inflammatory response characterizes the initial phase: presence of M1 macrophages, release of pro-inflammatory cytokines, proteolysis, and phagocytosis (4). In the second phase, the pro-inflammatory response tapers off (but doesn’t fully resolve), and the healing and remodeling processes begin (4). The number of reparative immune cells, such as M2 macrophages and regulatory T cells (Tregs), increases, contributing to the development of a mature scar (4). Persistence of the inflammatory response beyond the initial phase often results in pathological remodeling of the myocardium and contributes to heart failure (4).
MI pathophysiology also includes reperfusion-induced sympathetic nerve damage (5,6). And chondroitin sulfate proteoglycans in the cardiac scar activate protein tyrosine kinase phosphatase receptor-s on sympathetic nerves and prevent nerve regeneration even in the presence of nerve growth factor (5). In the end, the extent of nerve damage predicts the development of ventricular arrhythmias (7–9).
Previous studies have identified 3 novel therapeutics that can promote sympathetic reinnervation in the heart after MI: Intracellular sigma peptide (ISP), HJ-01, and HJ-02 (6,10,11). ISP prevents the binding of chondroitin sulfate proteoglycans to protein tyrosine phosphatase-s, and HJ-01 and HJ-02 disrupt protein tyrosine phosphatase-s–tropomyosin kinase A interactions (6,10,11). As a result, sympathetic nerves can regrow even in the presence of chondroitin sulfate proteoglycan. In addition, disrupting protein tyrosine phosphatase receptor-s normalizes heart function and confers resistance to isoproterenol-induced arrhythmias (6).
Sepe et al. conducted experiments to validate previous findings and determine the impact of treatments with ISP, HJ-01, and HJ-02 on the profile of the immune cells after MI. They hypothesized that therapeutics would shift the immune response into a reparative phenotype and used quantitative multiplex immunohistochemistry (mIHC) to test their hypothesis.
Mice underwent 2 surgeries: The first one to implant telemetry electrodes in the heart, and the second one, 5 days later, to induce ischemia-reperfusion. For the latter, researchers occluded the left coronary artery for 45 minutes and then released the ligature to induce reperfusion. Sham mice underwent surgery but had no occlusion. Between 3–10 days after surgery, mice received daily intraperitoneal injections of ISP, HJ-01, HJ-02, or vehicle. Data collection took place 14–15 days after surgery.
As previous studies have shown, treatment with ISP, HJ-01, and HJ-02 promoted sympathetic reinnervation of the heart, as assessed by immunohistochemistry (IHC) for tyrosine hydroxylase (5,6). Mice that received treatment had sympathetic nerve density similar to that of sham animals. In addition, sympathetic reinnervation of the heart resulted in functional improvement. Mice that received treatment had fewer isoproterenol-induced arrhythmia than mice that received vehicle.
Next, researchers used mIHC to phenotype immune cells in the heart after MI. They performed 15 rounds of IHC to target a total of 23 cell markers. Each round of the mIHC process consisted of antigen retrieval, incubation with primary and secondary antibodies, staining with ImmPACT® AEC Substrate Kit, Peroxidase (HRP), slide visualization and image acquisition, and stripping (Figure 1). The subsequent image analysis process took place in 3 steps: Image processing, cell classification, and quantification of cell types (Figure 1).
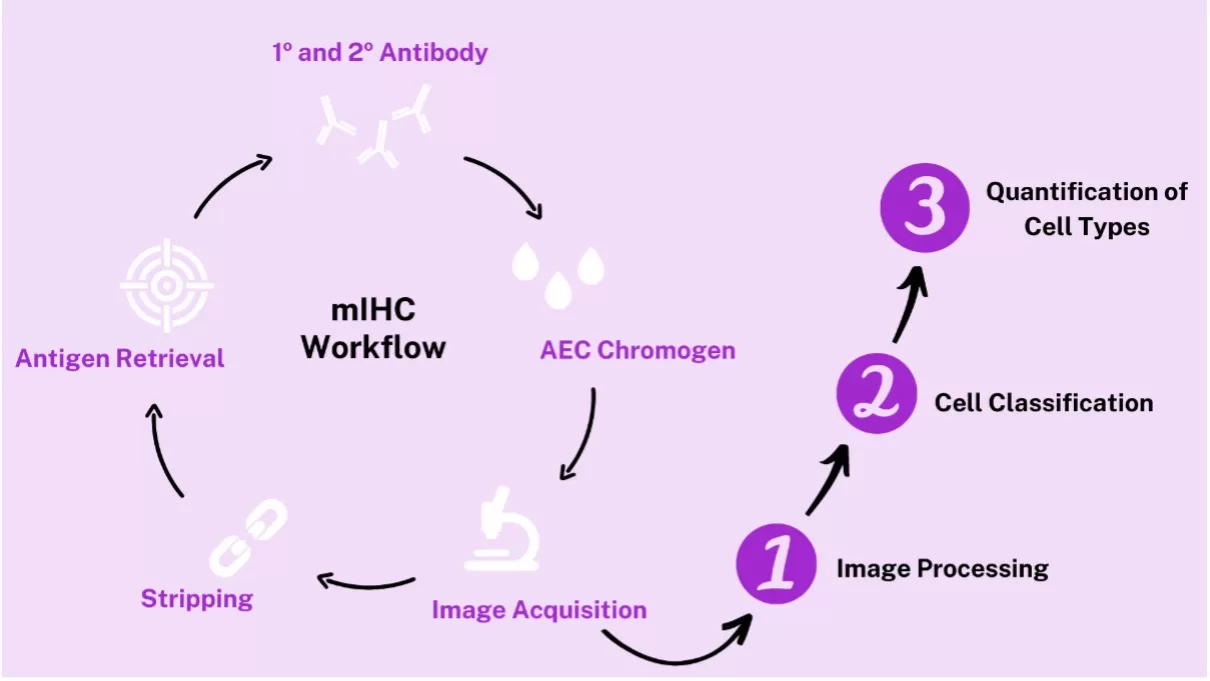
Using mIHC, Sepe et al. observed significant differences in the phenotype of immune cells between groups. Mice treated with ISP and HJ-02 had a higher ratio of M2/M1 macrophages and more Tregs than mice that received vehicle. Changes were more prominent in mice that received HJ-02 than in mice that received ISP. In addition to promoting reinnervation, treatment dampened the pro-inflammatory response and stimulated cells associated with the healing and remodeling processes. However, in vitro incubation with ISP and HJ-02 induced no change in peritoneal macrophage phenotype, suggesting that changes in phenotype require the synergistic effect of different factors.
Treatment with HJ-01 and HJ-02 also reduced infarct size, a change that wasn’t observed in mice that received ISP. Likewise, HJ-01 and HJ-02, but not ISP, improved cardiac output and ejection fraction. Finally, researchers observed that treatment-induced pathophysiological changes did not associate with changes in vascularization.
Data from this study provided insight into the mechanisms underlying the impact of therapeutics that disrupt protein tyrosine phosphates receptor-s on MI pathophysiology. After MI, glycans present in the cardiac scar bind to those receptors and prevent reinnervation of the heart. But treatment with novel therapeutics led to heart reinnervation. Using mIHC, researchers also identified that treatment shifted the immune response profile: The number of cells that support tissue repair and functional recovery increased. Future research that assesses later time points and determines the impact of reinnervation on the risk of heart failure will provide valuable information for developing new therapies to treat MI.
If you want to read about other scientific advances and learn tips and tricks to improve your processes and results, check out other pieces in the blog.





Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.