Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
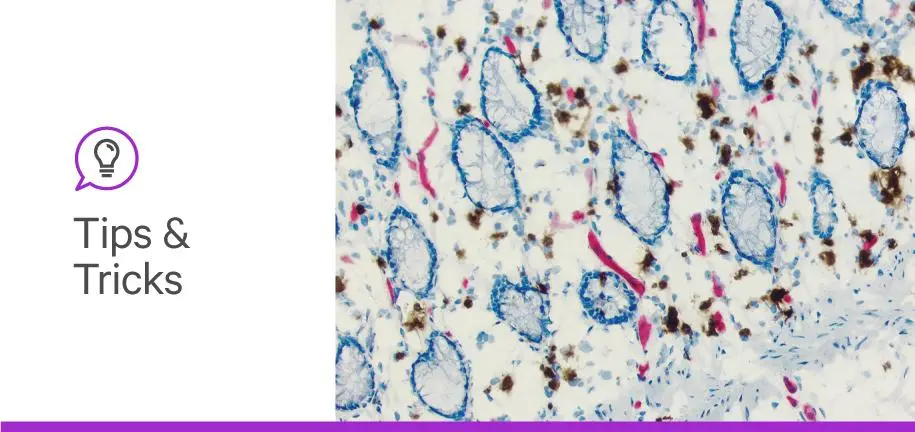
Performing a successful immunohistochemistry (IHC) or immunofluorescence (IF) experiment requires the perfect combination of primary antibody, secondary antibody, and detection system. Although IHC and IF are common techniques, researchers often struggle with them due to the complexity of biological specimens. When performing these experiments, scientists aim to reduce background or non-specific staining on the specimen. Identifying sources of non-specific staining is crucial for determining possible solutions to improve your staining results.
This blog post will briefly walk you through commonly encountered sources of non-specific staining and background in IHC and IF applications. If you’re interested in more in-depth knowledge as well as solutions to the sources listed below, check out our Blocking Guide.
Many detection kits use secondary antibodies conjugated to reporter enzymes, such as horseradish peroxidase (HRP) and alkaline phosphatase (AP), that convert chromogenic substrates into colored precipitates. Alternatively, reporter enzymes can also be conjugated to the primary antibody. In IHC applications, many different substrates can be used in combination with both HRP or AP to generate different colors. Some tissues have high endogenous peroxidases (e.g., blood-rich tissues such as the spleen and kidney) and phosphatase (e.g., kidney, intestine, and liver) (1). These endogenous enzymes can react with chromogenic substrates, resulting in high background in IHC applications.
Note: This is not relevant with standard IF assays.
Biotin, also known as vitamin B7, is a cofactor for some reactions that mostly occur in the mitochondria and is essential for the metabolism of fatty acids, glucose, and amino acids. Tissue types with higher mitochondrial activity, such as kidney, liver, spleen, and certain tumors, have high levels of endogenous biotin. Avidin is a glycoprotein with a natural affinity for biotin, and the strong non-covalent bond between them has allowed the development of many assays, including ABC (Avidin Biotin Complex) staining kits. Streptavidin is another naturally occurring protein isolated from Streptomyces avidinii that also binds to biotin and is used in detection systems. The primary difference between avidin and streptavidin is that the latter is not glycosylated and does not bind to endogenous lectins. However, both avidin and streptavidin can bind to other macromolecules inside the cell through direct affinity (e.g., endogenous biotin) or non-specific binding due to charge/ionic interactions, leading to non-specific staining.
If any of the detection antibodies in an IHC or IF procedure is directed against the species of a chosen specimen, undesired binding to endogenous immunoglobulin can occur and obscure the desired staining. Common examples of this scenario include the use of mouse primary antibody on mouse tissue (mouse on mouse staining) and human or humanized primary antibodies on human tissue sections (human on human staining), which are subsequently detected with an anti-mouse or anti-human secondary antibody respectively.
Cross-reactivity occurs when either the primary or the secondary antibody binds to an unintended epitope. Primary antibodies frequently bind to non-target sites in the specimen during the incubation process. These non-specific interactions rarely affect the final IHC results because they are weak and usually break during subsequent washes. However, when the primary antibody concentration is too high, non-specific binding of primary antibodies to non-target sites can persist, resulting in high background (2,3). Although primary antibodies can bind to non-target sites, cross-reactivity is more commonly observed between secondary antibodies and unintended epitopes on the sample, leading to non-specific staining.
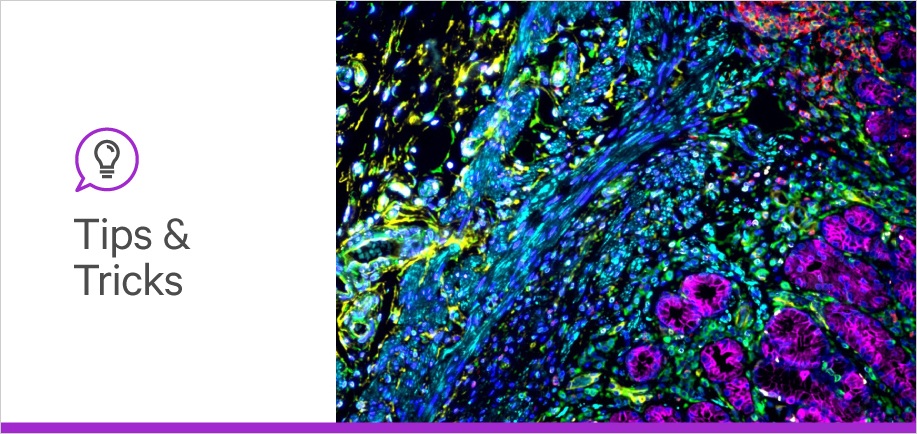
Autofluorescence is a very common potential cause of background in IF. It occurs when molecules other than the antigen-bound antibody complex emit fluorescence. Common natural sources of autofluorescence include the heme groups of red blood cells, collagen, elastin, NADH, and lipofuscin. Autofluorescence from these sources makes the interpretation of IF results challenging, particularly when using the green and red channels (4-6). The fixation process can also contribute to autofluorescence as formalin-induced cross-links can emit fluorescence in a broad range of wavelengths (4).
Note: This is not relevant for standard enzyme-based assays.
Now that you know some potential sources of background and non-specific staining, you can take the right steps to resolve these issues and improve your staining results. Be sure to check out the Blocking Guide for staining solutions as well as our blog for more tips and tricks.
References




Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.