
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.

Post-translational modifications, such as glycosylation, play a critical role in a myriad of cellular functions. As the product of glycosylation, glycoconjugates are responsible for vital cellular functions from proliferation to immune response. These functions are mediated by lectins, the family of proteins that recognize glycans with high specificity. Their glycan-specific nature also makes them important tools that can help profile, characterize, and capture complex glycans in biological systems.
While antibodies are used to detect and identify proteins, plant lectins have a few advantages over them when it comes to glycan analysis. You can use lectins to identify not only glycan expression patterns but also cell types based on known glycan expression patterns. Other advantages of plant lectins include wide availability, ease of extraction, stability, and, of course, their glycan-specific nature.
We cannot talk about glycobiology without mentioning monosaccharides, the building blocks of carbohydrates.
The best part is that they can be incorporated into a broad range of protein identification tools, where typically you might use antibodies for antigen detection. If you are a newbie in glycan analysis, applying lectins to conventional assays might seem perplexing. Join us as we walk through the key considerations to help you get started.
Let’s review three commonly used applications where you can easily leverage lectins for glycan analysis:
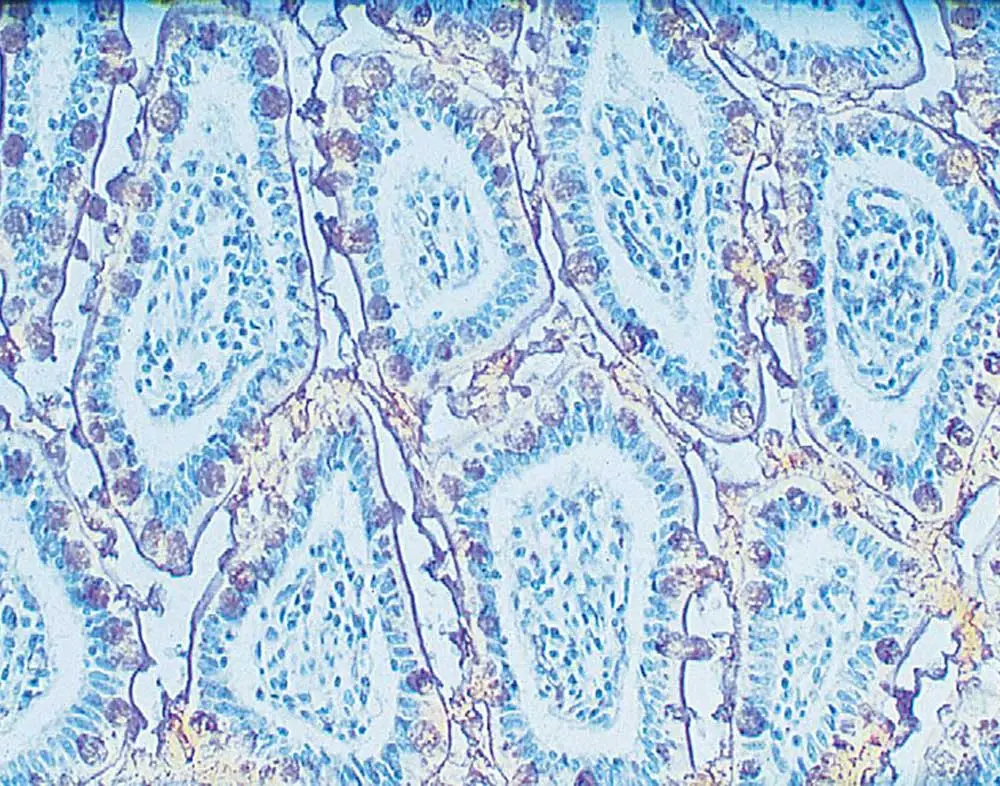
IHC is one of the most useful applications for characterizing cell-surface glycoproteins, identifying and categorizing cell types based on glycan expression, and predicting behavior. IHC helps us reveal insightful information about surface glycoconjugates, such as their location and movement in the cell, relative intracellular vs. extracellular abundance, and spatial orientation. In addition, differential staining allows a clear view of the tissue morphology with respect to the glycan expression patterns. Finally, because of the use of enzyme-based approach, tissue staining is more durable—sometimes lasting even years—making IHC the preferrable approach for long-term studies. Numerous properties of lectins in IHC have enabled researchers to identify and monitor glycan alterations in pathological processes, such as tumor differentiation (1) and pathogenic infections (2).
The lectin IHC workflow begins with the usual tissue preparation and fixation steps. However, instead of using primary antibodies to detect the antigen of interest, you will incubate samples with biotinylated or unconjugated lectins. Keep in mind that similar to IHC with antibodies, it is essential to optimize the amount of lectin you use to avoid agglutination and high background.
The primary binding is followed by the binding of secondary reagents (e.g., streptavidin-peroxidase, labeled antibodies, etc.) specific to the lectins bound to your glycoconjugates. The secondary reagents are usually coupled to enzymes that catalyze reactions leading to colored products (aka chromogens).
And just like regular IHC with antibodies, you can visualize lectin expression via brightfield microscopy. To generate better contrast and accentuate the primary stain, other biomolecule-specific counterstains can be administered to stain the remaining parts of the tissue.
IF exhibits some similarities to IHC in terms of the information it can reveal, such as the distribution and abundance of aberrant carbohydrate structures in cancer cell compartments (3). However, instead of using chromogens to generate color, you conjugate lectins to fluorophores. These are fluorescent molecules (mostly dyes), which absorb light at a short wavelength and, upon excitation, re-emit light at a longer wavelength. The resulting lectin conjugate can be visualized under a fluorescence microscope.
Aside from revealing spatial information. IF offers the added advantage of multiplexing to help you to differentiate between different targets even when their fluorescent labels have a spatial overlap. While you can also perform multiplexing with IHC, it is easier to see coexpression/colocalization with IF.
Instead of primary antibodies, you will need to incubate your cells with lectins that specifically bind the target glycoconjugate. There are two methods of IF, depending on how you want to attach your fluorophore. In direct (primary) IF, the glycan-specific lectin itself carries the fluorophore. For indirect IF, a secondary reagent carries the fluorophore (e.g., streptavidin fluorophore) that specifically recognizes and binds the lectin.
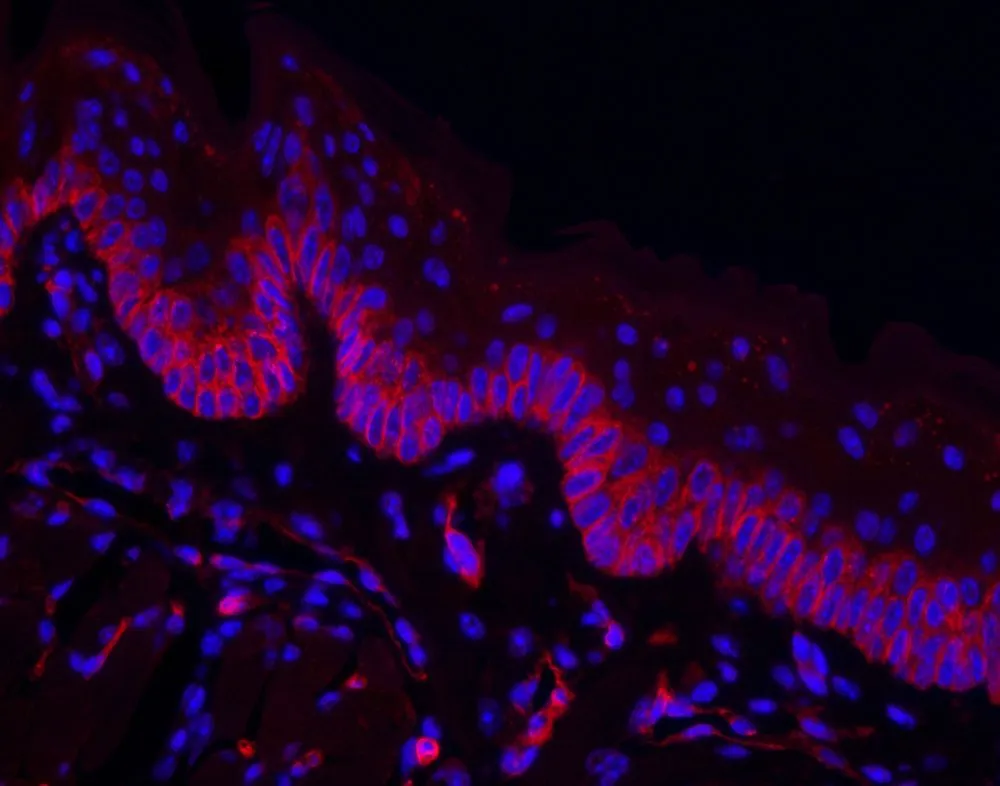
In the indirect IF method, biotinylated lectins are more preferable because the non-covalent interaction between biotin and the streptavidin fluorophore amplifies the signal. Furthermore, you can apply streptavidin/biotin blocking techniques to eliminate potential background issues from endogenous biotin.
Both IF methods have their pros and cons. While the direct staining method involves fewer steps and is quicker, it is less sensitive because lectins have a limited number of fluorescent molecule conjugation sites. Fluorophore conjugation to lectins is restricted by the limited number of internal lysine residues and N-terminal amino groups available that can be targeted by amine-reactive crosslinkers, e.g., NHS esters. On the other hand, indirect staining with multiple steps can amplify fluorescence. A two-step method using biotinylated secondary antibody and fluorescent streptavidin can be further amplified: you can apply an additional layer of biotinylated anti-streptavidin and fluorescent streptavidin to incorporate more fluorophores and significantly increase the signal-to-noise ratio. However, this enhanced sensitivity adds more time to your protocol than does direct fluorescent staining and increases the risk of background staining.
Note: An important caveat in both IHC and IF is that lectins can weakly bind to the reactive sites of off-target proteins, which can create background staining. Therefore, it is crucial to reduce non-specific lectin binding by using blocking buffers during incubation. For antibody-based assays, you can use a variety of suitable buffers, such as normal serum and gelatin. However, these buffers could contain glycoconjugates, which could competitively inhibit specific lectin binding (4). Use of a Carbo-Free Blocking solution is a more suitable alternative for lectin applications because—as its name suggests—it is free of glycoconjugates.
Flow cytometry can be used to measure and analyze the glycan structures of cells by suspending them in a fluid that passes through a laser beam. Despite the lack of spatial information, flow cytometry enables you to achieve a rapid view and analysis of the cell population.
Although flow cytometry exhibits similarity to IF in its use of fluorescence-conjugated lectins, it differs in the type of results it generates. While IF can illustrate the intracellular distribution of glycan structures, flow cytometry instead quantifies the same glycan structures. In flow cytometry, fluorophore-conjugated lectins (instead of antibodies) can bind specific glycan structures and emit light in characteristic wavelengths that help distinguish the different glycan structures. For example, flow cytometry can reveal differential glycosylation states between healthy and tumor-associated cells, making it a vital tool for cancer glycobiology (5).
Unlike IF, flow cytometry can handle a much larger sample size, meaning you can scan hundreds of thousands of cells in reasonably short time frames. This makes flow cytometry incredibly useful in situations where you have heterogeneous cell populations. By grouping the cells according to the intensity of the fluorescent signal, you can sort cells with respect to glycan expression levels. This kind of cell sorting makes further analysis considerably more practical, especially when you want to identify and isolate rare cell populations. One example is encountered in stem cell engineering, where researchers can employ flow cytometry with lectins to group stem cell lineages and isolate human neural progenitor cells (6).
When leveraged appropriately, lectins are simple yet powerful for harvesting valuable information about glycans and their involvement in various diseases.
Vector Laboratories offers a variety of unconjugated or conjugated lectins for various lectin-based assays. In addition, we offer a comprehensive lectin guide that illustrates lectin workflows for different applications. Not only will you gain access to step-by-step workflow specifics, but you can also read more about the history of lectin research and current studies that apply lectin-based assays.
Click here to download our Lectin Application and Resource Guide.
References





Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.