
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Glycans are the key to numerous biological processes from cellular uptake to recognition, but their proper functioning depends on interactions with other macromolecules. In particular, cell surface glycans can mediate a cascade of cellular processes upon recognition by carbohydrate-binding proteins, such as antibodies and lectins. Although the mechanism of action behind glycan recognition is yet to be fully understood, what we have learned so far has enabled us to utilize these molecules to isolate, visualize, and interpret glycans. Lectins particularly became an effective research tool by the end of the 20th century. Their initial discovery involved immunohematology studies investigating how specific plant-derived proteins, hemagglutinins, could agglutinate red blood cells. Hemagglutinins from different seed extracts were found to agglutinate 1 of the blood types A, B, and O with no interaction with the other 2 (1). In the 1950s, Morgan and Watkins attributed differential agglutination to the hemagglutinins recognizing sugar chains on red blood cell surfaces (2). The ability of these proteins to distinguish between sugars granted them the term lectin, derived from the Latin legere, which means “to select” (3).
Today, the prevalence of lectins in glycan-related biological activities is widely accepted. Subsequent studies led to the discovery of endogenous lectins binding the organism’s own surface glycans. It also became clear that lectins were found not only in plants but also in other kingdoms, including animals.
There are, in fact, multiple ways of categorizing lectins based on their structures or the glycan chains that they preferentially bind. The most high-level classification involves the species of origin since lectins are found in various organisms. Lectins from different kingdoms differ not only in their binding properties and requirements but also in the applications they are used in.
Algal lectins are used widely in biomedical cancer and HIV research since they were found to exhibit anticancer and antiviral activity (4). While many lectins require a divalent cation for binding, algal lectins are exempt from this requirement. Furthermore, they usually have an affinity for glycoproteins instead of standalone monosaccharides.
Fungal lectins are involved in the growth and development of fungal species, as well as in their symbiotic relationship with plants (5). This process, called mycorrhization, allows fungi to exchange resources with plants and depends on plant glycan recognition by fungal lectins. They are mainly found in mushrooms and, to a smaller extent, in microfungi, yeast, and mycelia (6).
Bacterial lectins can bind surface glycans at the terminal sugar or the internal oligosaccharide chains. You might recall from our Introduction to the Universe of Glycans blog post about the function of glycans that bacteria use the host surface glycans as a vehicle to invade the host cell. As expected, bacterial lectins, a.k.a adhesins, are crucial to this attachment since they recognize specific glycan sequences on their hosts (7). Besides pathogenic interactions, bacterial lectins play a critical role in symbiosis (8).
Animal lectins bind soluble glycoconjugates on cell surfaces, serving many functions, such as signaling pathways, cell-cell adhesion, regulation of protein levels in the blood, and immune defense against pathogens (9). They comprise carbohydrate recognition domains with a specific amino acid sequence while also requiring a divalent cation for the binding. Animal carbohydrate recognition domains (CRD) sequences usually contain 115–130 amino acid residues (10). In the next section, we will explore how animal lectins are categorized according to these sequences.
Plant lectins contribute not only to the development of plants but also to their interactions with the ecosystem. They are mainly responsible for establishing symbiosis with nitrogen-fixing bacteria while protecting plants against pathogenic microorganisms (11).
Plant lectins have sparked particular interest in biomedical research due to their abundance in nature and ease of isolation. More importantly, they were found to possess non-catalytic domains that reversibly bound specific glycan sequences. The majority of lectin-related research is geared toward plant lectins, with more than 500 types having been isolated by the end of the 20th century (12).
Animal lectins exhibit great diversity in their carbohydrate-binding domains, which makes sense considering the diversity of glycans they need to recognize to carry out a large number of bodily functions. Through crystallization studies, scientists have characterized CRD sequences and grouped them according to similarities in CRD sequence motifs and structural properties. The 4 main types of animal lectins are C-type, P-type, I-type, and S-type, although recent studies have led to the discovery of other classes, such as M-type, L-type, chitinase-like, and F-type lectins.
C-type lectins are animal lectins known for their Ca2+ dependence for binding glycans. They depend on Ca2+ to coordinate their amino acid residues to be able to bind the hydroxyl groups of sugars more easily. Found in macrophages, dendritic cells, and endothelial cells, C-type lectins are responsible for bacterial pathogen recognition, clearance, triggering of cytokine production, and other immunomodulatory processes that prepare the body for parasitic attacks.
P-type lectins are highly specific towards mannose-6-phosphate. Some P-type lectins can have a single extracellular domain with a cation-independent site, while others have large extracellular domains with 2 Ca2++-requiring binding sites. These intracellular transmembrane proteins often facilitate the transport of intracellular enzymes, such as lysosomal enzymes, from the Golgi apparatus to other compartments.
Also called galectins, S-lectins are characterized by their homologous S-carbohydrate recognition domains (S-CRD) with multiple glycan-binding sites and conserved cysteine residues, having specificity for β-galactoside ligands. They play a crucial role in cell growth, adhesion, and migration by interacting with the glycans in the extracellular matrix.
I-type lectins share a common motif with immunoglobulin (Ig)–like domains that contain 2 β-sheets. These β-sheets comprise 70–110 amino acids each and are linked by several hydrogen bonds and a disulfide bond. The majority of I-type lectins bind sialic acid and are called sialic-acid binding immunoglobulin superfamily lectins (Siglecs). Their sialic-acid recognition sites enable them to bind sialic acid-containing ligands in natural killer (NK) cells, B cells, and T cells. That’s why they are hugely responsible for recruiting immune cells.
Plant lectins are perhaps the best-studied ones owing to their wide distribution and ease of extraction. The broad scope of plant lectin research has also led to a more elaborate classification system. In fact, it is possible to classify plant lectins in 3 different ways.
Plant lectins can possess more than 1 carbohydrate-binding site. That’s why they are divided into 4 categories based on the number of binding sites:
Another way to classify plant lectins is by the crystal structures of the CRD, similar to the classification of animal lectins. Here, we come across 7 groups:
The third category is based on their binding affinity to specific carbohydrate moieties, which is why this grouping system is the most suitable for glycan profiling and quantification.
That said, initial categorization was incomplete due to relying only on oligosaccharides and disaccharides as binding moieties instead of more complex epitopes. A recent study by Mahal et al. proposes a more precise way to profile plant lectin specificity (13). The study used a machine learning algorithm and glycan microarray data to investigate binding specificity for 57 plant lectins, each of which fell into 1 of the following categories:
On a side note, although each lectin exhibits preferential binding to a specific glycan epitope, its specificity could be altered with the substitution or addition of glycan residues. For example, Phaselus Vulgaris-L (PHA-L) is a complex N-glycan Binding lectin with specificity towards β1,6-branched N-glycans; however, this specificity is reduced upon α2-6 sialylation.
In addition, some plant lectins can contain additional binding motifs. For example, the sialic acid-binding Maackia amurensis-II (MAL-II) predominantly binds α2,3-sialylated Galβ1−3GalNAc in O-glycans, but it also can recognize a 3’O-sulfated galactose epitope.
Our “Importance of Lectin Specificity” blog post summarizes the binding properties of various plant lectins mentioned in the study. Solid knowledge of these plant lectin classes helps researchers make informed decisions about commercial plant lectin purchases to carry out glycan microarray studies. For example, the National Center for Functional Glycomics (NCFG) at Harvard University offers microarray resources based on their glycan specificity.
Regardless of the species they are found in, lectins serve as companions for glycans, triggering a multitude of biological phenomena and maintaining a livelihood. Their responsibilities range from cell growth and development to cell adhesion and migration. In addition, they can recognize pathogenic cell surface glycans and trigger immunogenicity. Meanwhile, certain lectin classes—particularly plant lectins—are useful tools to elucidate the biological roles of glycans.
We hope our blog post provided you with the fundamentals of lectins. Additional resources can help you build on the basics. PubMed is home to hundreds of review papers and original studies demonstrating the broad spectrum of lectin applications in life sciences. Furthermore, the National Center for Functional Glycomics at Harvard University provides a comprehensive list of lectins from Vector Laboratories and Sigma Aldrich, with data encompassing certificates of analysis and datasheets from the CFG and the NCFG QA/QC arrays. Detailed explanations of our lectin products can be found in the Vector Laboratories Lectin Resource Guide and Lectins Infographic. To learn how plant lectins from Vector Laboratories contribute to glycobiology research, stay tuned to our SpeakEasy Science Blog.
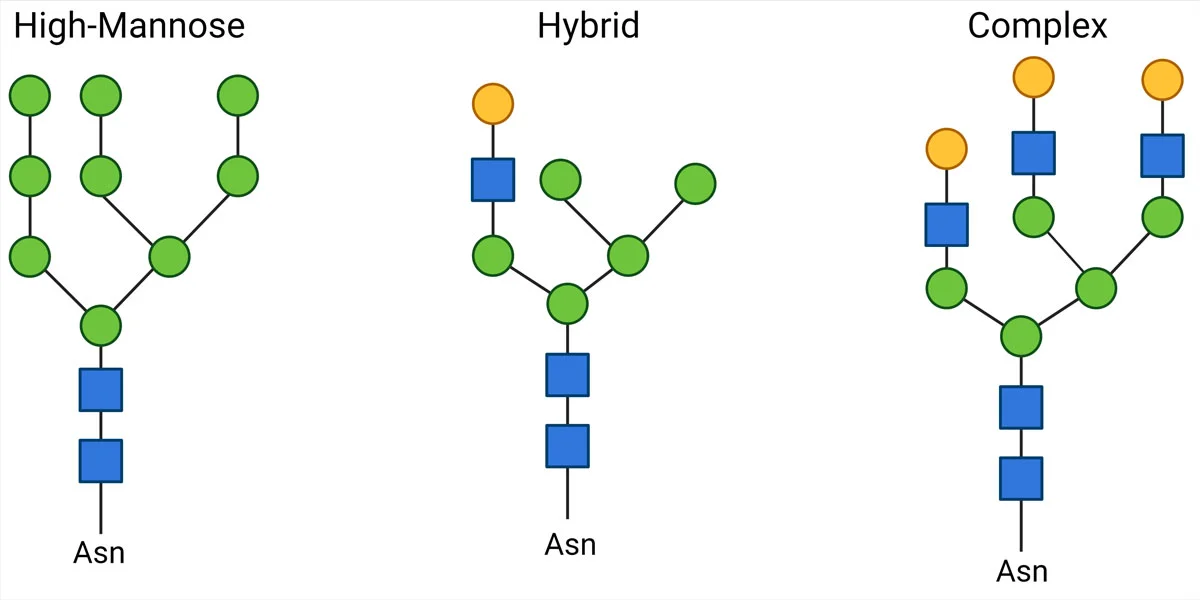
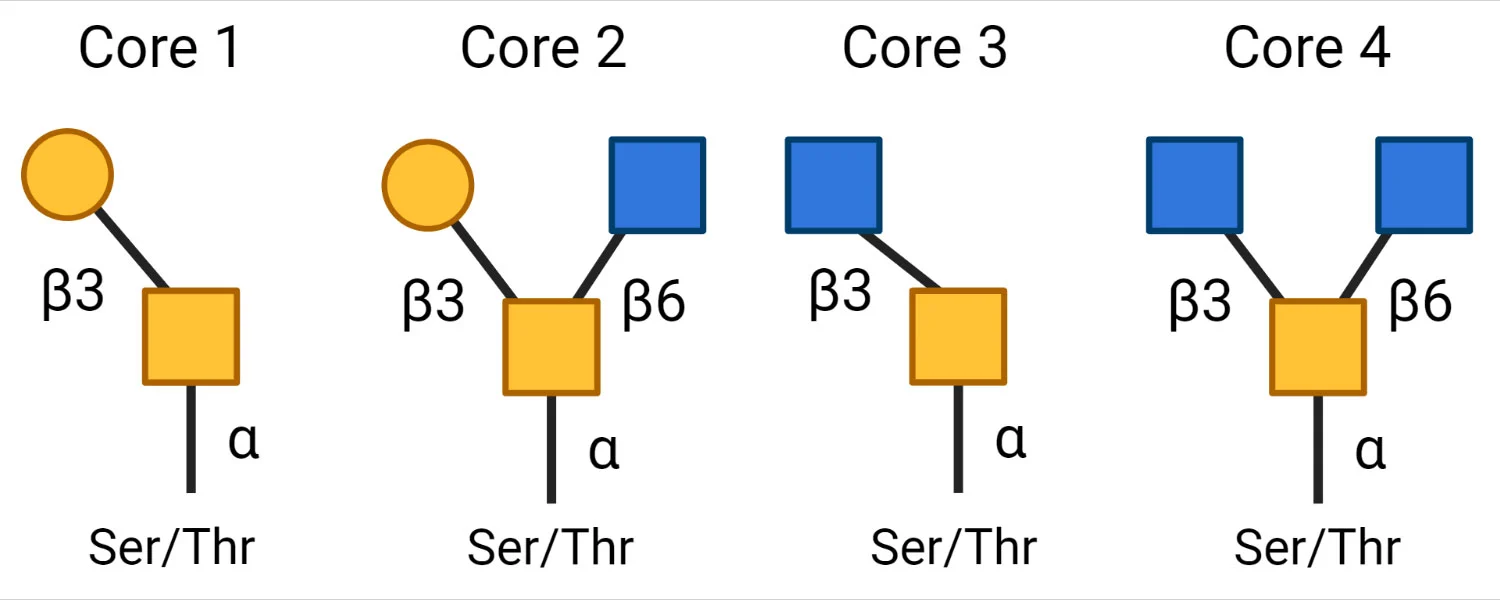
O-linked glycans are oligosaccharides attached to the oxygen atom of the side chains of Serine or Threonine residues. We can further classify O-glycans according to the sugar residue attached to the peptide.
In many O-glycosylated proteins, mainly membrane glycoproteins, the first sugar to attach is N–acetyl-galactosamine (GalNAc). Although there is no specific residue sequence preference for glycosylation, as there are several GalNAc transferases with different site preferences, O-glycosylation sites are often close to a proline residue.
Subsequently, different core structures are possible depending on the next monosaccharide. The 4 most common core structures are as follows:

Sugar residues, such as galactose, GlcNAc, sialic acid, and fucose, can be added to the core structures by glycosyltransferases.
Other O-glycans include O-GlcNAc, O-Mannose, O-galactose, and O-glucose, although they are less common than O-GalNAc. In particular, O-GlcNAc is found on cytoplasmic and nuclear proteins in the cell. The O-glycosylation of intracellular proteins is less diverse than that of extracellular glycoproteins. One theory is that extracellular glycosylation evolved to be more diversified to facilitate the binding of various pathogens and glycan-binding proteins.
Glycosaminoglycans are long and linear polysaccharides with a repeating disaccharide unit, where a galactose residue or a uronic sugar (containing carbonyl and carboxylic acid groups, e.g., glucuronic acid or iduronic acid) is attached to an amino sugar (where 1 hydroxyl group is replaced with amine -NH2, e.g., GlcNAc or GalNAc).  There are 4 classes based on their repeating core units, with each type involving a different synthesis mechanism.
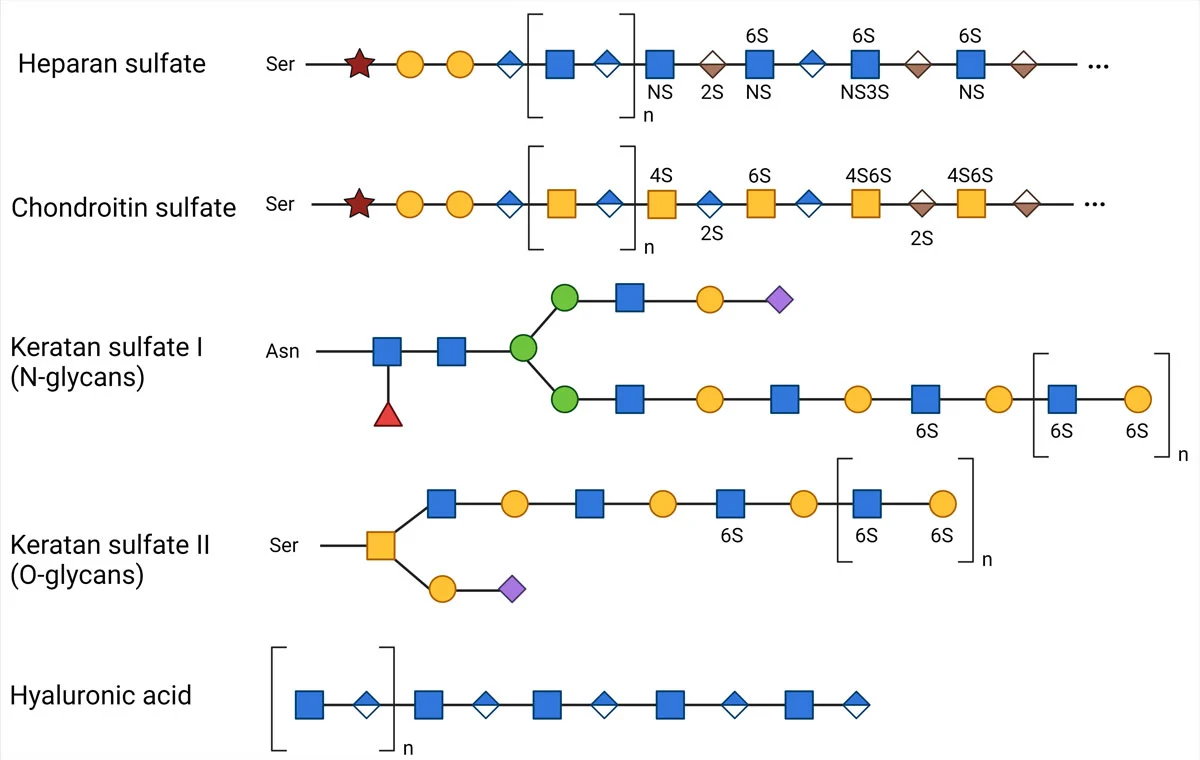

The synthesis of GAGs starts in the cytoplasm, where 5 uridine diphosphate (UDP) derived active sugars are formed. With the exception of Hyaluronic Acid, all GAG precursors undergo sulfation in their functional groups, which takes place in the Golgi apparatus. In contrast, the precursor sugars of Hyaluronic Acid are transferred directly to the cell membrane.
Besides proteins, glycans can also be covalently attached to lipids to form glycolipids crucial to cell membrane integrity, cell-cell interactions, and immune response. Glycolipids are classified according to their hydrocarbon backbone: glycerolipids with glycerol and sphingosine with sphingolipids.
It is nearly impossible to attribute a specific function to a glycan or glycan subclass. The same glycan structure can serve a range of tasks across different organisms as well as at different locations of the same organism. That’s why we present a broadened and simplified classification of glycan function.
Glycans are ubiquitous in cellular compartments, membranes, and extracellular matrix, where they alter the structure and function of the proteins and lipids that they are attached to. Therefore, glycans play a critical part in shaping primary macromolecule structures, contributing to the integrity of cells and cellular components. Glycosylated macromolecules are paramount to a long list of structural and modulatory functions, from protection and transport to site-specific functions, such as lubrication of the joints to prevent friction (1).
Perhaps the most well-known example is the mucin peptide, which forms protective layers on the epithelial cell surfaces to protect tissues against pathogens and free radicals (2). In fact, aberrations in cell surface glycosylation are often associated with inflammatory diseases and cancer. Another example of a protective function is that provided by cellulose (3) and chitin (4), glycan polymers made of glucose and GlcNAc, respectively, which are indispensable components of cell walls that provide strength and stability for plants and fungi.
Glycan-induced structural modifications can also alter the physical properties of macromolecules to confer advantages. Blood plasma is the first example to come to mind since it contains a high concentration of glycoproteins, where hydrophilic glycans augment protein solubility (5). In addition, glycosylation alters the charge density of proteins, thereby preventing their degradation by proteases (6).
Many organisms tend to form different forms of symbiotic relationships with one another, and it has become evident that cell surface glycans are one of the main control points of these interactions.
Terminal glycan residues are particularly critical to pathogen-host interactions. For instance, parasitic bacteria, including E. coli (7), and Helicobacter (8), adhere to the host cells by recognizing cell surface sialoglycans. Another example is malaria, where P. falciparum invades red blood cells by binding specific sialic acid residues on the surface (9).
Extrinsic recognition also applies to viral infections, including the most recent pandemic caused by COVID-19. A growing body of evidence suggests that N– and O-glycosylation of the spike protein strengthens its binding affinity to the ACE2 receptor while helping it evade the host immune system (10).
Fortunately, extrinsic recognition is bidirectional, meaning that host immune systems combat pathogens through glycan recognition. More specifically, glycan-binding proteins (which we will cover in the next blog post in more detail) can recognize the surface glycans of pathogens to entrap and destroy them.
Cell-cell communication and cellular trafficking are greatly influenced by glycan recognition. Cellular uptake mechanisms, such as endocytosis and phagocytosis, are often mediated by the cell surface receptors that recognize macrophage terminal glycans. This allows cells to pull in molecules from outside the cell, transport material across organelles, and discard unwanted material.
Essential processes in organelles are also mediated by glycan receptors. For example, receptors in the ER carry out specific folding patterns for the incoming proteins based on their glycan epitopes. By the same logic, incorrectly folded proteins can be detected and discarded upon recognizing aberrant glycan structures.
Cell-cell interactions also rely on glycan recognition by surface proteins and are crucial for the survival of individual organisms and the harmony of entire ecosystems. Well-characterized examples include glycosylated fibrinogen-induced blood clotting (11) and egg-sperm interactions (12). On a larger scale, glycan-mediated intercellular signaling between rhizobacteria is largely responsible for establishing their symbiotic relationship with plants for nitrogen fixation, which has a large impact on the food chain (13).
Pathogens that often evade innate immune reactions decorate their cell surfaces with glycan structures found on host cell surfaces (14). This means that pathogens can camouflage their antigenic epitopes behind these glycans. This can be achieved through various mechanisms, such as the appropriation of host glycans or convergent evolution of the pathogen, enabling the mimicry of the biosynthetic pathways of the target host.
The study of glycans can provide us valuable insights about their roles in our biological systems. While there is an overwhelming diversity in glycan structure and function, we believe that this article recaps the fundamentals of structural diversity and the functional significance of glycans.
That said, there are several resources for you to expand your knowledge of glycan biochemistry. In the article “Sweet systems: technologies for glycomic analysis and their integration into systems biology”, Chen, et al. explains glycan function with examples from the 4 classes of functions. The article also discusses the current setbacks and questions in glycobiology. For more specific carbohydrate related data, you can check out GlyGen: Computational and Informatics Resources for Glycoscience. Last but not least, our SpeakEasy Science Blog and Glycobiology eBook serve as a scientific hub where you can find practical information about glycan detection and quantification as well as reviews of peer-reviewed articles exploring the roles of glycans in cancer.





Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.