
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.

There are many important factors to consider as you go through the process of preserving your pathological or histological tissue sections. If we asked you to name the most important, chances are the mounting medium wouldn’t be at the top of the list. Things like successful sectioning and choosing the right stain are probably higher up on your list of concerns. But, after all of the care you take to generate your stained specimen, don’t you want to do everything you can to ensure you can take a picture-perfect image?
That’s where the right mounting medium comes in. The last step in the staining workflow, mounting is necessary to ensure the tissue section can be viewed under a microscope. Further, it enables the specimen to be stored for later viewing. The mounting medium is the substance in which the specimen is embedded and provides a uniform and optically clear environment for imaging of the specimen (1). Choosing the right mounting medium for your tissue can make or break a successful tissue preservation process as the appropriate medium will prevent your specimen from drying out, preserve its integrity for long-term storage, and closely match the refractive index of the glass slide for high-quality, high-magnification imaging. The wrong one… Well, you get the idea.

Mounting media generally fall into two categories: water (aqueous)-based, or solvent (non-aqueous)-based liquids. Water-based mounting media allow you to mount directly from aqueous buffers and provide a suitable pH for sample preservation, as well as the viscosity needed to easily apply a glass coverslip. Within this category, there are two subsets. Non-setting, water-based media stay in a liquid state, which means you may need to use a sealing material, such as nail polish or paraffin wax, around the perimeter of the coverslip to prevent motion. The advantage of a non-setting medium is that the refractive index is stable at all time points (don’t worry, we’ll explain later) and microscopic viewing can occur as soon as the sample is prepared. Setting, water-based media, as you might imagine, form a solid film upon drying (generally, within 1-4 hours) and do not require any extra sealant. The refractive index, however, may require up to 24 hours to completely stabilize.
The use of solvent-based, or resinous, mounting media goes back to the 1830s, when Canada balsam, a resin made from a balsam fir tree, was first used. The vast majority of solvent-based mounting media are setting and will solidify with time, sealing the coverslip and the sample. Generally referred to as permanent mounting media, they will preserve and protect the sample with an optically clear film for years. However, when using these mounting media, it is important to consider that the samples cannot be mounted directly from aqueous buffers since the mounting medium is not miscible with water. In most cases, the slide must be transferred through a series of dehydration steps, removing the water from the specimen and converting the sample’s environment to a non-aqueous one. Usually, a series of dehydration (ethanol) and clearing (xylene) solutions are used to accomplish this.
At the most basic level, any aqueous buffer could serve as a mounting medium. However, the refractive index (RI) of water (1.33) differs significantly from that of the glass slide and coverslip (1.51), oil-immersion liquids (1.51), and the tissue section itself (1.38–1.46) (2). Because of this, using water as a mounting medium can result in RI mismatching. With mismatching RIs, focal point broadening, or spherical aberration occurs, which can cause resolution degradation and affect sample brightness (3). That’s why matching the RI of your mounting medium to the RI of the glass components (and immersion liquid, when used) will optimize the clarity, resolution, and brightness of the images.
Many water-based mounting media use glycerol as the major component since the RI of glycerol (1.47) is close enough to glass to allow high quality imaging, while the final dried film of a solvent-based mounting media will generally have an RI of 1.45–1.49, which also allows for high-quality imaging.
It can take a bit of practice to prepare mounted slides without any air bubbles, but, generally, there are two methods for coverslipping tissue sections: the slide method and the coverslip method. In the slide method, you apply a small amount of the mounting medium onto the tissue section, place one end of the coverslip on the slide, slowly lowering the coverslip with the aid of a dissecting needle or forceps, and then allow the slide to air dry in a horizontal position. Going slowly is the key to this method as it makes air bubbles less likely to form. In the coverslip method, rather than adding the mounting medium directly onto the tissue, you instead apply it to the center of the coverslip. You then slowly lower the inverted slide until you make contact with the coverslip, letting the surface tension pull the coverslip up, invert the slide quickly, and allow it to air dry in a horizontal position.
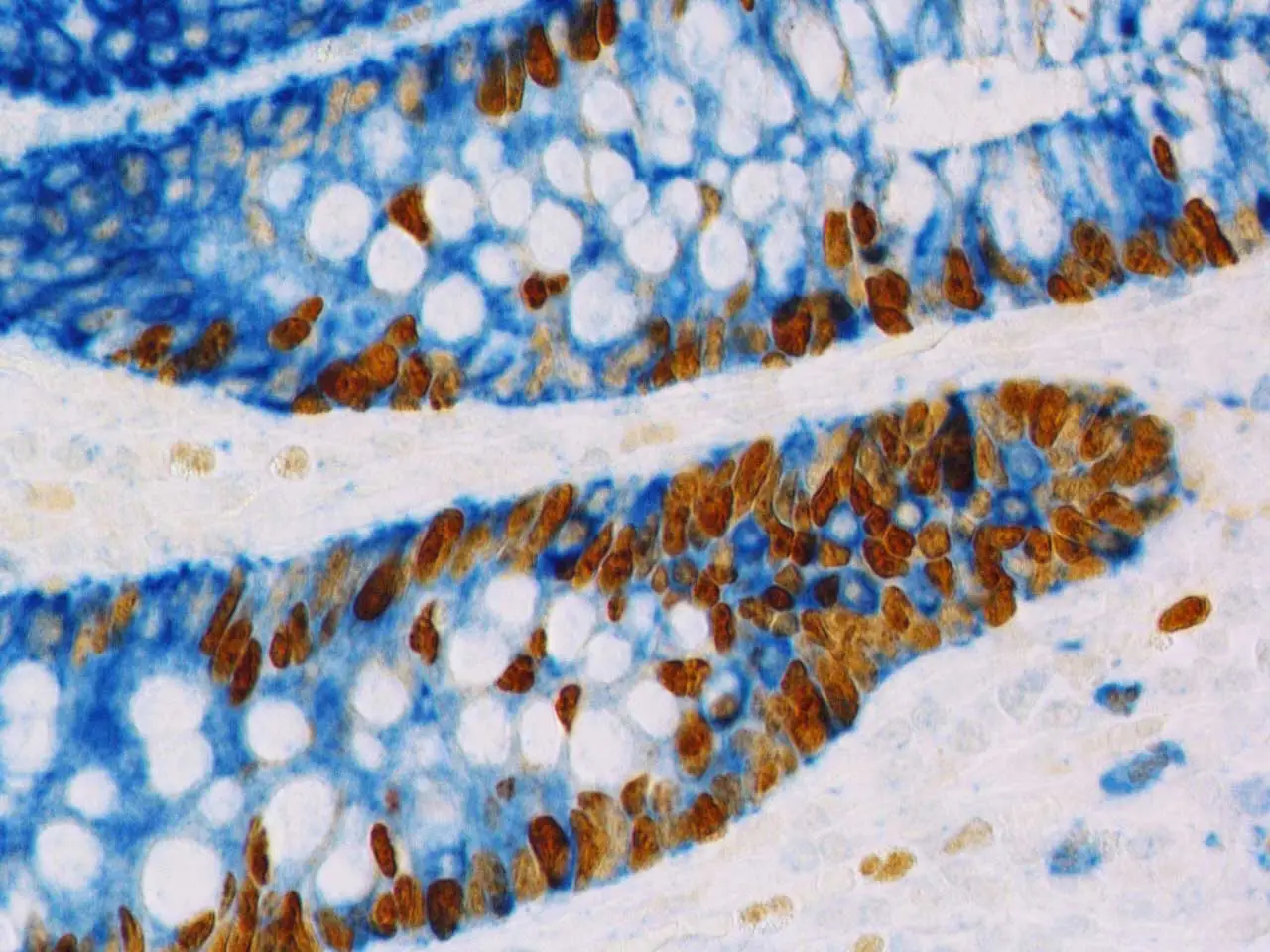
When using immunohistochemical (IHC) enzymatic deposition (such as DAB) and tissue counterstains (such as hematoxylin, eosin, methyl green), there are some additional requirements for an effective mounting medium. To preserve the integrity, color, and crispness of enzymatically deposited substrates and histological stains, you need to make sure that your chosen medium can prevent diffusion and fading while maintaining the color consistency of your stain or substrate.
Vector Laboratories offers three IHC mounting media that are optimized for use with Vector Laboratories Substrate Kits and Counterstains.
Maximizing the signal brightness and stability of fluorophores under illumination requires the use of an aqueous mounting medium; the vast majority of fluorescent molecules used in biomedical assays are optimized for aqueous environments. Further, the medium should be antifade, preventing the fluorescently labelled antibody or fluorescent protein from photobleaching (i.e., fading) under illumination. Photobleaching occurs upon high magnification viewing under laser or lamp illumination due to processes that occur with photoexcited molecules. While some of these processes are reversible, most cause permanent damage and quenching to fluorescent molecules. The mechanisms that lead to photodegradation of fluorescent molecules are both oxygen-dependent and oxygen-independent processes (4).
Using an antifade medium, however, is akin to applying sunscreen to your fluorescent detection molecules. It contains antioxidant molecules that react with photoexcited molecules, preventing the photoinduced damage that causes fluorescent molecules to fade. Vector’s family of VECTASHIELD® mounting media, in particular, are designed to produce the highest signal intensity for the most commonly used fluorophores while providing the greatest protection available to prevent photobleaching.
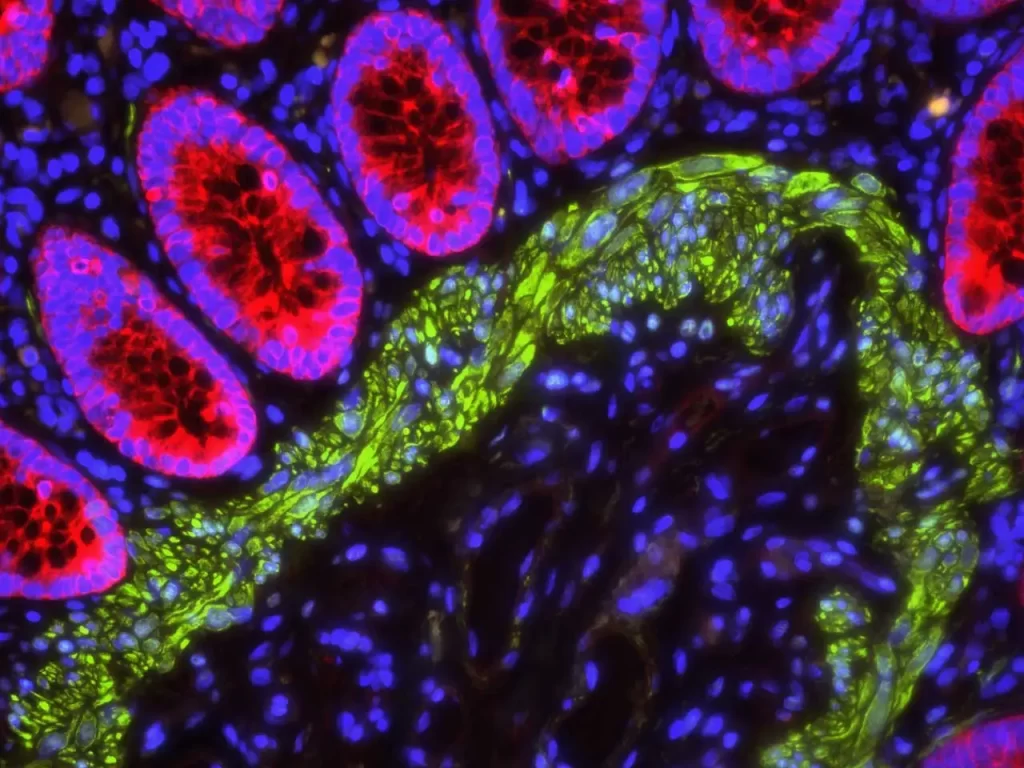
As you can see, there’s a lot to consider when it comes to choosing the right mounting medium, but, now that you know what to look for, you can move forward with confidence and find the best medium to fit your research needs.
References





Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.