
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
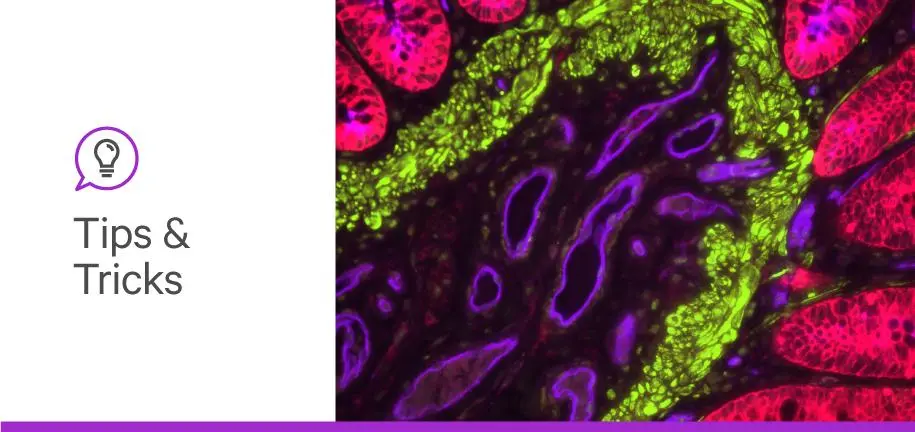
Glycans and their bioconjugates are paramount to our livelihood, mediating numerous cellular processes and interactions. By the same logic, changes in glycan networks play a central role in many diseases, from cancer to autoimmune disorders. So why is it that only a fraction of commercially available biomarkers emphasizes aberrant abnormal cell glycome? When you think about all the possible combinations of simple sugars that can bind to each other at various sites, you realize the astonishing diversity of glycan structures that can form on the cell surface. That’s why analysis of complex glycomes was intimidating until the discovery of carbohydrate-binding proteins—a.k.a. lectins.
The word lectin derives from the Latin word legere, which means “to choose”, referring to their ability to recognize and bind to distinct carbohydrate structures. The question is, why does this feature make lectins such promising tools for glycobiology?
As biomarker detection tools continue to evolve, new ways of utilizing lectins are starting to emerge. This article summarizes how lectins are beneficial as glycan research tools. Knowing the doors lectins can open will help you reap the benefits of their unique biological functions. Keep reading for insights, and be sure to also check out our Glycobiology resource page for more on lectins.
To elicit biologically-relevant information about aberrant glycosylation, you need to visualize cell glycan distribution and its impact on morphology. Fortunately, lectins are compatible tools for molecular imaging protocols, such as immunohistochemistry (IHC) and immunofluorescence (IF), as the tissue staining protocols for antibodies are mostly valid for lectins. Both workflows are simple to implement, producing rapid results.
In your IHC workflow, you can conjugate a lectin to enzymes (e.g., horseradish peroxidase) or haptens (e.g., biotin). The resulting lectin conjugate and appropriate substrate can be viewed under light microscopy or electron microscopy.
Your other option for your IF workflow, label lectins with a fluorescent dye (e.g., fluorescein isothiocyanate (FITC)), which allows you to view glycan distribution with a fluorescent microscope. The use of lectins in IF has the added advantage of multiplexing, which allows you to use multiple fluorescent dyes to visualize multiple types of glycan chains in the same sample.
Scanning a sample for multiple glycan sequences can be time consuming without high-throughput analysis methods. Lectins can provide time-saving and cost-effective analysis with the help of very small solid surfaces, such as microchips.
Because you want a comprehensive glycan profiling of your sample, you need to detect as many different glycan structures as possible. Compared to antiglycan antibodies which have stringent specificity towards glycans, making them more time-consuming to use, lectins are more versatile in their binding patterns, meaning that one lectin can bind multiple glycans (1). This makes lectins more effective than antibodies as microarray tools.
One of the advantages of lectins is their ability to detect glycans without the need to release them from their bioconjugates. Lectin blotting, based on western blotting, is a method that provides insight into glycan structures while they are still attached to their glycoproteins. Intact glycoproteins or glycolipids can be probed with lectins and there is no requirement for cleaving the whole complex. This also makes lectin blotting a promising tool for comparing glycosylation networks between control and test samples. For instance, researchers could demonstrate altered sialylation and fucosylation of N-glycans in colorectal cancer by comparing biofluid samples from cancer patients to those from healthy subjects (2).
Lectin microarray is another method for rapid glycan detection that does not require glycan release. This allows you to detect and differentiate all possible disease-associated glycan isomers, aberrant sialic acid linkages, and terminal glycan structures within glycoproteins and glycolipids. For example, lectin microarray was used extensively to help researchers discover predictive biomarkers in several cancer types, such as colorectal (3) and gastric cancer (4).
Understanding the correlation between specific glycan structures and cell characteristics is necessary for elucidating cell size, proliferation, and differentiation. To obtain biologically-relevant data, you should be able to work with live cells. The great news is that you can integrate fluorescently labelled lectins into flow cytometry protocols to perform live-cell imaging.
The use of lectins in flow cytometry is advantageous because it offers an opportunity for quantitative analysis of glycan profiles in different cell subtypes. Lectins can help characterize cellular subpopulations even when a specific cell type is scarce in the overall cell population.
The combination of lectins and flow cytometry led to many breakthroughs in stem cell research. Using lectins in flow cytometry, researchers were able to successfully characterize human embryonic stem cells (hESCs) and their differentiated progeny based on lectin-binding profiles. This also allowed them to isolate neural progenitor cells to analyze their role in brain development in detail (5).
Imagine wanting to analyze a specific aberrant glycoprotein in a sample, but the concentration of that particular glycoprotein is low. The solution is to run affinity chromatography to obtain eluates enriched with the glycoprotein of your interest.
In lectin affinity chromatography, you can utilize the carbohydrate specificity of lectins by immobilizing them on your chromatography surface or matrix. Upon washing, the immobilized lectin binds the corresponding glycoprotein while the rest of the sample is washed away. The resulting elution is now suitable for mass spectroscopy and proteomics, generating further insight into the glycosylation mechanism of your protein. For example, lectin affinity chromatography played a significant role in the enrichment of core fucosylated peptides that are potential biomarkers in pancreatic cancer (6).
The unique chemical and physical properties of lectins make them ideal candidates for studying cell-surface glycans and their impact on complex diseases. Their integration into primary glycan analysis techniques can accelerate your glycan analysis while generating consistent and reliable data.
There are numerous ways to identify and analyze cellular glycans with lectins, so it might be difficult to determine the most appropriate method for your specific needs. At Vector Laboratories, our aim is to inform and support you with detailed explanations and demonstrations of lectin workflows. To learn more about lectin applications, you can visit our Lectins resources page, where you can find tools that will help you incorporate these powerful molecules into your workflow.
1. Masarova J, et al. 2004. Optical Lectin Based Biosensor as Tool for Bacteria Identification.” Polish Journal of Microbiology.
2. Qiu Y, et al. 2008. Plasma Glycoprotein Profiling for Colorectal Cancer Biomarker Identification by Lectin Glycoarray and Lectin Blot. Journal of Proteome Research.
3. Nakajima K, et al. 2015. Establishment of New Predictive Markers for Distant Recurrence of Colorectal Cancer Using Lectin Microarray Analysis. Cancer Medicine.
4. Futsukaichi T, et al. 2015. Decreased Expression of Bauhinia Purpurea Lectin is a Predictor of Gastric Cancer Recurrence. Surgery Today.
5. Dodla MC, et al. 2011. Differing Lectin Binding Profiles Among Human Embryonic Stem Cells and Derivatives Aid in the Isolation of Neural Progenitor Cells. PloS One.
6. Tan Z, et al. 2015. Large-Scale Identification of Core-Fucosylated Glycopeptide Sites in Pancreatic Cancer Serum Using Mass Spectrometry. Journal of Proteome Research.





Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.