Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
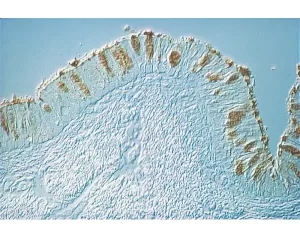
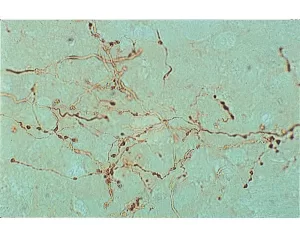
This lectin binds oligomers of N-acetylglucosamine and some bacterial cell wall oligosaccharides containing N-acetylglucosamine and N-acetylmuramic acid. Although the carbohydrate binding specificity is similar to wheat germ agglutinin and Datura stramonium lectin, several differences have been reported for potato lectin.
Biotinylated Solanum tuberosum lectin has an appropriate number of biotins bound to provide the optimum staining characteristics for this lectin. This conjugate is supplied essentially free of unconjugated biotins and is preserved with sodium azide.
| Unit Size | 2 mg |
|---|---|
| Applications | Immunohistochemistry / Immunocytochemistry, Immunofluorescence, Blotting Applications, Elispot, ELISAs, Glycobiology |
| Recommended Usage | For most applications we recommend a freshly prepared working solution of 5-20 µg/ml in the below buffer. |
| Recommended Storage | 2-8 °C; Store frozen for long term storage |
| Solution | 10 mM HEPES, pH 7.5, 0.15 M NaCl, 0.08% sodium azide, 0.1 mM CaCl2, 20 mM N-acetylglucosamine. |
| Concentration | 2 mg active conjugate/ml |
| Conjugate | Biotinylated |
| Sugar Specificity | N-Acetylglucosamine |
From our experience we have found that some lectins require Ca++ to be present for optimal binding activity. We suggest using calcium chloride (CaCl2) to fortify working solutions and ensure a minimum level of Ca++ is met. This may be particularly pertinent if using phosphate based buffers as diluents and storage solutions.
Solanum tuberosum lectin consists of two identical 50 kDa subunits. The subunits can dissociate in solution to produce a monomeric form of the lectin which does not agglutinate cells.
This biotinylated lectin is an ideal intermediate for examining glycoconjugates using the Biotin-Avidin/Streptavidin System. First the biotinylated lectin is added, followed by the VECTASTAIN ABC Reagent, Avidin D conjugate, or streptavidin derivative.
Inhibiting/Eluting Sugar: Chitin Hydrolysate
Applicable patents and legal notices are available at legal notices.


Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.
How do I Request a Quote?
To request a quote for products: