Description
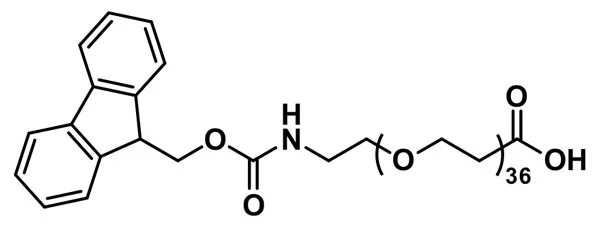
“Fmoc-N-amido-dPEG®36-acid, product number QBD-10903, is one of a broad line of products designed for use in peptide synthesis. The long (112 atoms), flexible, discrete PEG (dPEG®) spacer is functionalized with a propionic acid group on one end and Fmoc-protected amine on the other. The compound can be added to the N-terminus of a growing peptide chain or to a primary-amine-functionalized side chain of an amino acid such as lysine. The non-immunogenic dPEG®36 spacer increases the hydrodynamic volume and imparts water solubility to the conjugate molecule.
QBD-10903 permits our customers to insert a dPEG® spacer into a peptide chain using familiar Fmoc chemistry using solid phase or solution phase chemistry. However, due to the spacer’s length, it may work better in solution-phase syntheses. The dPEG® compound can be inserted at either end of the peptide chain or in the middle of two amino acid sequences to provide a flexible linker between distinct functional peptides. Additionally, the dPEG® spacer can be used to provide spacing in a synthetic construct where steric hindrance is a problem. The amphiphilic nature of dPEG® products means that the construct gains hydrodynamic volume and water solubility while remaining soluble in organic solvent. The Fmoc protecting group is removed easily with a solution of piperidine in N,N-dimethylformamide (DMF).”
References
Greg T. Hermanson, Bioconjugate Techniques, 3rd Edition, Elsevier, Waltham, MA 02451, 2013, ISBN 978-0-12-382239-0; See Chapter 18, Discrete PEG Reagents, pp. 787-821, for a full overview of the dPEG® products.
Lipo-Oligomer Nanoformulations for Targeted Intracellular Protein Delivery. Peng Zhang, Benjamin Steinborn, Ulrich Lachelt, Stefan Zahler, and Ernst Wagner. Biomacromolecules. 2017, June 26, 2017. DOI: 10.1021/acs.biomac.7b00666.
Flexible antibodies with nonprotein hinges. Daniel J. Capon, Naoki Kaneko, Takayuki Yoshimori, Takashi Shimada, Florian M. Wurm, Peter K. Hwang, Xiaohe Tong, Staci A. Adams, Graham Simmons, Taka-Aki Sato and Koichi Tanaka. The Japan Academy, Series B. 2011, 87 (9) pp 603-616. November 11, 2011. DOI: 10.2183/pjab.87.603.
A Systematic Analysis of Peptide Linker Length and Liposomal Polyethylene Glycol Coating on Cellular Uptake of Peptide-Targeted Liposomes. Jared F. Stefanick, Jonathan D. Ashley, Tanyel Kiziltepe, and Basar Bilgicer. ACS Nano. 2013, 7 (4) pp 2935–2947. February 19, 2013. DOI: 10.1021/nn305663e.
Enhanced Cellular Uptake of Peptide-Targeted Nanoparticles through Increased Peptide Hydrophilicity and Optimized Ethylene Glycol Peptide-Linker Length. Jared F. Stefanick, Jonathan D. Ashley, and Basar Bilgicer. ACS Nano. 2013, 7 (9) pp 8115–8127. August 29, 2013. DOI: 10.1021/nn4033954.
PEG-Peptide Conjugates. Ian W Hamley. Biomacromolecules. 2014, 15 (5) pp 1543-1559. April 1, 2014. DOI: 10.1021/bm500246w.
Evaluation of Nonpeptidic Ligand Conjugates for SPECT Imaging of Hypoxic and Carbonic Anhydrase IX-Expressing Cancers. Peng-Cheng Lv, Karson S. Putt, and Philip S. Low. Bioconjugate Chemistry. 2016, 27, pp 1762-1769. June 30, 2016. DOI: 10.1021/acs.bioconjchem.6b00271.
Bioresponsive nanocarries for targeted intracellular delivery of proteins and peptides. Ruth Elisabeth and Johanna Roder. Dissertation zur Erlangung des Doktorgrades der Fakultat fur Chemie und Pharmazie der Ludwig-Maximilians-Universitat Munchen. 2016, pp 1-128.
Efficient capture of circulating tumor cells with low molecular weight folate receptor-specific ligands. Yingwen Hu, Danyang Chen, John V Napoleon, Madduri Srinivasarao, Sunil Singhal, Cagri A Savran, Philip S Low. Scientific Reports. 2022. May 20, 2022. https://doi.org/10.1038/s41598-022-12118-3