Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
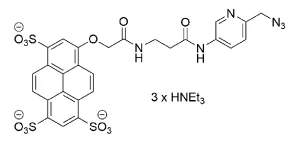
AZDye™ 800 Picolyl Azide is an advanced fluorescent probe that incorporates a copper-chelating motif to raise the effective concentration of Cu(I) at the reaction site to boost the efficiency of the CuAAC reaction, resulting in a faster and more biocompatible CuAAC labeling. Up to 40-fold increase of signal intensity, compared to conventional azides, was reported (see Selected References).
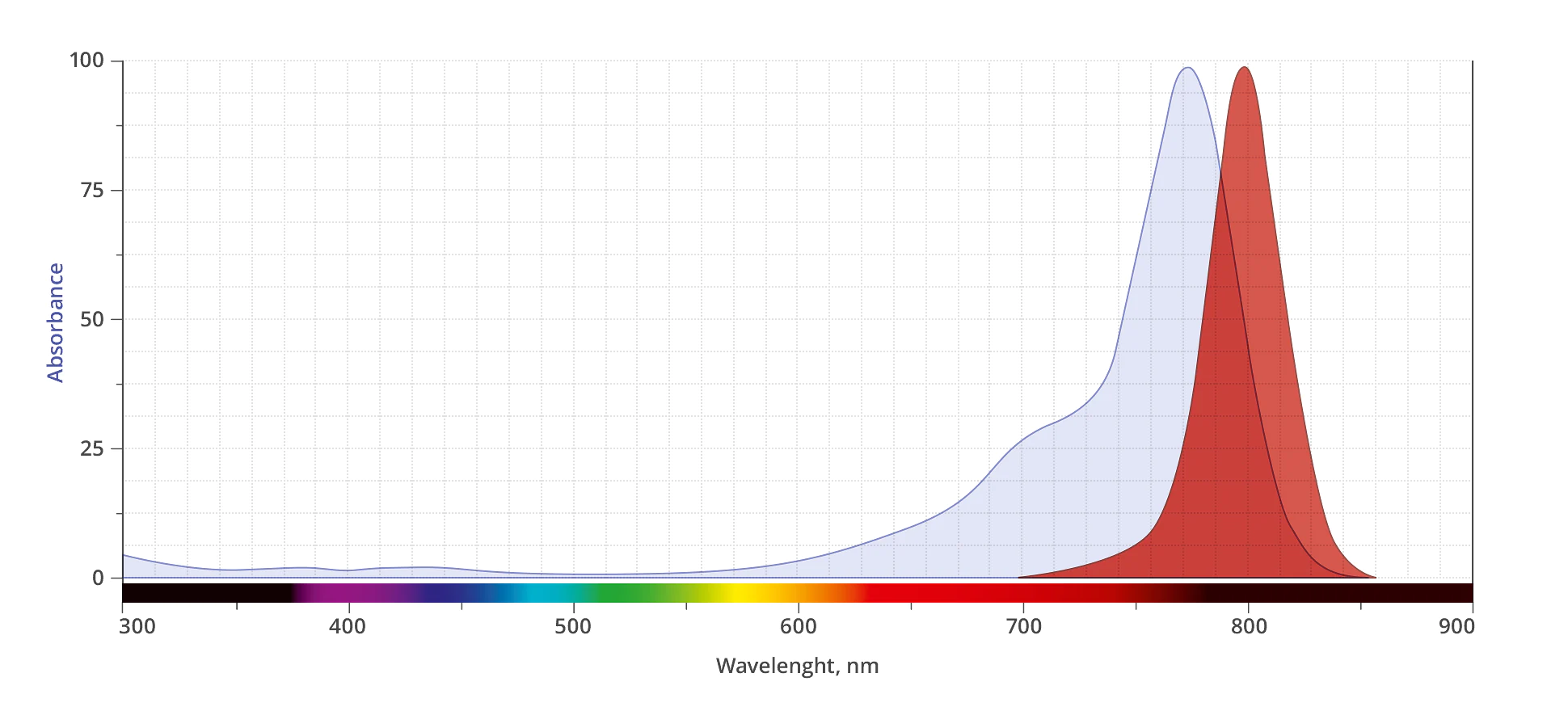
AZDye™ 800 is a conceptually new class of sterically shielded NIR cyanine heptamethine dye. This dye contains two shielding PEG arms directly over both faces of the heptamethine fluorochrome blocking any undesired bimolecular association processes and thus dramatically enhances the fluorescence brightness. Its excitation ideally suited for the 800nm channel of imaging systems. AZDye™ 800 is spectrally is almost identical to IRDye 800CW and DyLight 800.
Cyanine heptamethine dyes are well-known NIR fluorophores, with emission wavelengths >740 nm, and often are reagents of choice for labeling antibodies. Two of the most popular commercial NIR cyanine heptamethine dyes for antibody conjugation are IRDye 800CW and DyLight 800. While these NIR dyes are undoubtedly useful for many types of immunofluorescence technologies, the resulting NIR dye-labeled antibodies sometimes exhibit performance limitations due to three inherent fluorophore concerns. (1) A meso-OAryl group connected directly to the heptamethine fluorochrome group is susceptible to nucleophilic displacement by biological amines and thiols1, 2 resulting in a diminished chemical stability of the dye-antibody conjugates during synthesis, storage, or the time-course of an imaging experiment. In addition, the electron-donating meso-OAryl group promotes high fluorochrome reactivity with electrophilic singlet oxygen, resulting in relatively poor dye photostability.3,4 (2) Both dyes are flat molecules with a hydrophobic core and a polyanionic charge periphery. Thus, the chemical conversion of a small polar, cationic lysine residue on the antibody surface to a large hydrophobic, polyanionic dye derivative has the potential to produce substantial changes in antibody folding and physiochemical properties, leading to lower antibody stability and decreased target specificity.4-8 (3) When activated versions of these hydrophobic dyes are conjugated to an antibody surface, they tend to attach at the proximal lysine sites as stacked face-to-face dimers, which produces a diagnostic H-dimer peak in the absorbance spectra that is nonfluorescent.8,9 Moreover, the close stacking of proximal conjugated IRDye 800CW or DyLight800 on an antibody surface amplifies the potential for a deleterious effect on antibody targeting because of a localized patch of polyanionic charge and hydrophobicity.
In order to address these drawbacks of commercially NIR cyanine a conceptually new class of sterically shielded NIR dyes was developed in Dr. Bradley D. Smith laboratory.10, 11 These dyes contain two shielding PEG arms directly over both faces of the heptamethine fluorochrome blocking any undesired bimolecular association processes and thus enhance the fluorescence brightness. A recently published antibody labeling study clearly demonstrated 1-2 order of magnitude increase in brightness compared to commercially available NIR dyes as a result of almost complete prevention of stacking of multiple fluorophores appended to the antibody surface.10, 11
These sterically shielded NIR dyes with greatly improved chemical and photochemical stability and substantially enhanced brightness will enable researchers to greatly improve various types of indirect NIR immunofluorescence imaging and diagnostics applications that require high sensitivity and also develop new photonintense techniques that require high photostability.
MB 800Z is a zwiteroinic, charge-balanced dye with an equal number of anionic sulfonate and cationic ammonium residues, a structural feature that is known to reduce interactions with off -target biological surfaces.
| Unit Size | 1 mg, 5 mg, 25 mg |
|---|---|
| Abs/Em Maxima | 775/799 nm |
| Extinction Coefficient | 204,000 |
| Spectrally Similar Dyes | IRDye® 800CW, CF® 800, DyLight® 800 |
| Molecular weight | 1621.83 (protonated) |
| CAS | N/A |
| Solubility | Water, DMSO, DMF |
| Appearance | Green solid |
| Storage Conditions | -20°C. Desiccate |
| Shipping Conditions | Ambient temperature |
Applicable patents and legal notices are available at legal notices.

Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.
How do I Request a Quote?
To request a quote for products: