
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.

Christian Bonatto Paese developed a passion for biology as a youth. He grew up on a small farm-like house where he took care of the chickens, ducks and rabbits with his grandparents and mother, an elementary school biology teacher who took her children along to some of her classes. He became fascinated with basic research in his first year in college and quickly gravitated toward the field of developmental biology. His academic journey started in Brazil, where he earned a bachelor’s degree in Biological Sciences (Western Parana State University) and a master’s degree in Cell and Developmental Biology (Federal University of Santa Catarina). He then went on to England, gaining his PhD in Molecular Sciences (Oxford Brookes University) and is now in the US for his postdoctoral research. After his first year as a Postdoctoral Visiting Fellow at the National Cancer Institute (Frederick, Maryland), he began a fellowship in the Division of Plastic Surgery at the Cincinnati Children’s Hospital in 2019 with the goal of developing pharmacological interventions for birth defects to replace the more invasive and repetitive surgical solutions that are available today.
“I love the US right now, but I never thought of coming here. I always thought that going to the US was only for the best of the best. Getting to the NIH, I saw a lot of people who had the same mentality that I did back in my early days, but we were just wrong. The NIH accepts people from all over the world, and it’s a very nice community.”
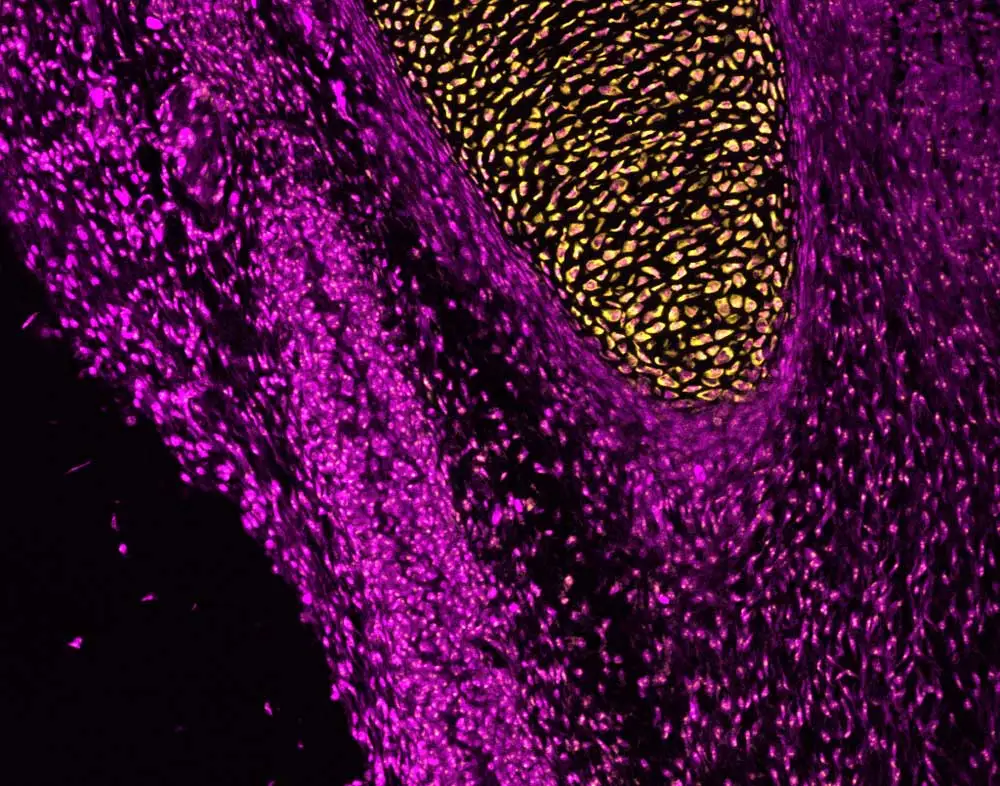
Christian’s research involves studying a defect in the microtubule organelles (cilia) in an avian mutant, talpid2 (ta2). In chicks, this ciliopathic mutation causes several phenotypes mostly in the craniofacial region that disrupt feeding, making this an excellent animal model to help better understand human development. In humans, the craniofacial phenotypes include debilitating birth defects, such as cleft lip/cleft palate (an opening in the lip and/or the roof of the mouth), micrognathia (smaller-than-normal lower jaw), craniosynostosis (premature fusion of bones in a baby’s skull), and others that often require surgery (or multiple surgeries) to repair. The ultimate goal of research in this area is to understand how these defects happen and then work to reduce the need for surgical intervention by replacing it with well-timed pharmacotherapeutic solutions. To accomplish these aims, a much better understanding of the developmental processes that contribute to craniofacial deformities is needed.
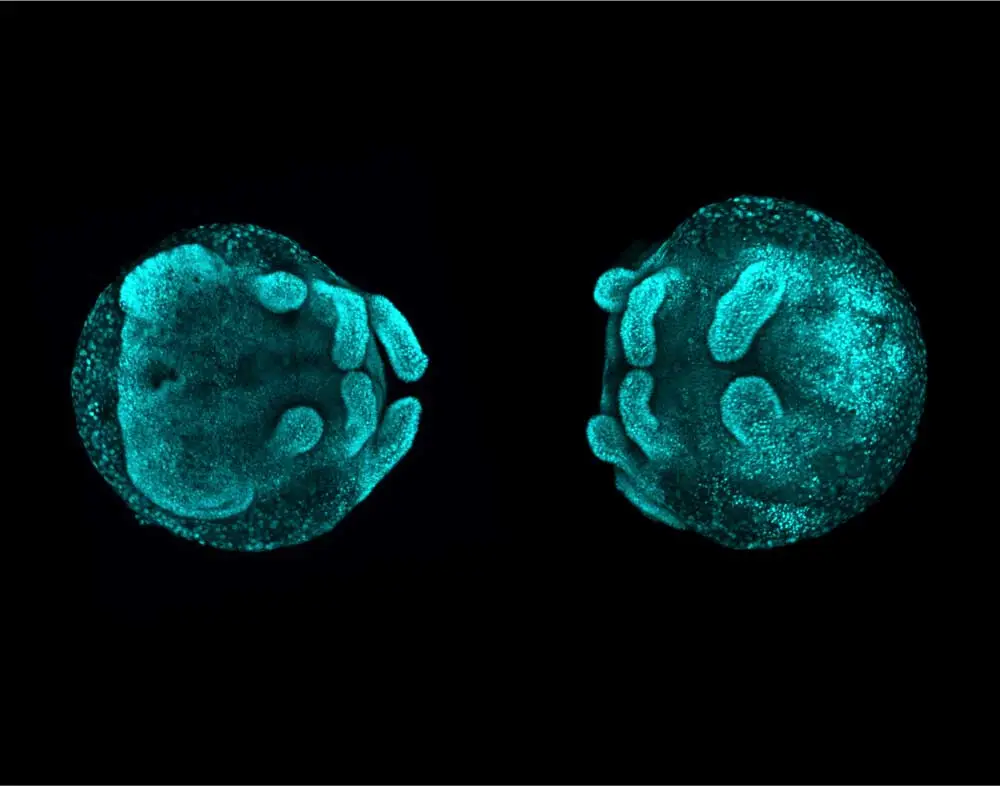
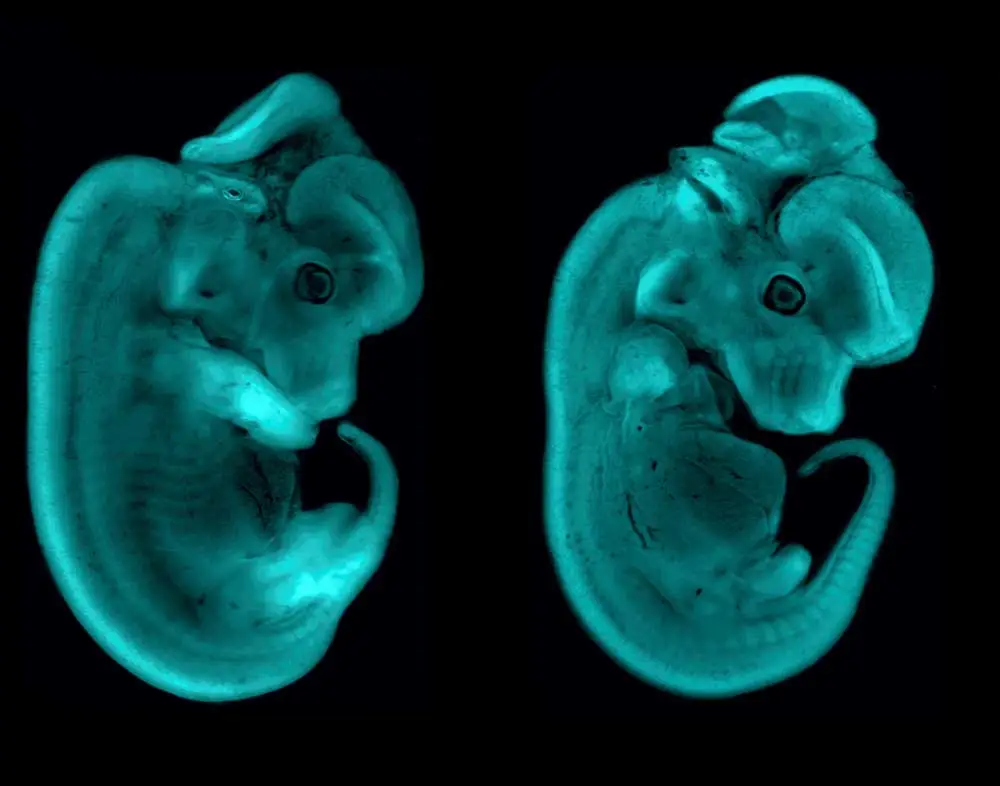
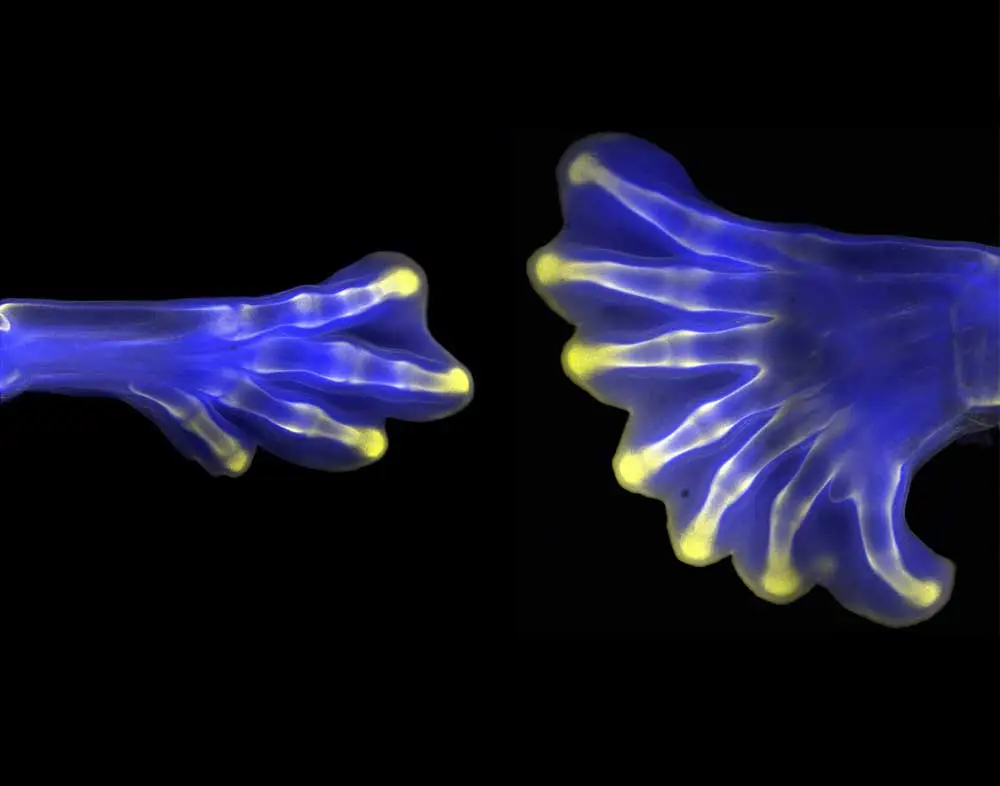
“Back in my master’s research lab, they were doing developmental biology in a crustacean model that is very important for the economy of that region in Brazil. At Oxford, we did developmental biology in a spider model to understand how body segmentation into the head, trunk, and abdomen works. At the NIH, I was working with mouse limb development, and right now I’m working with craniofacial development… to try to understand the biological developmental processes that are affected in birth disorders. We can use chicken, frog, and mouse models to make better treatments possible for these children. I have worked with four different animal models throughout my career, and the images that I’ve made along the way summarize my career. In all of them, I’ve worked with Vector Laboratories’ products.”
On a typical Wednesday, Christian receives a large box of chicken eggs that contain embryos with the mutation of interest, labels all the eggs, and transfers them into an incubator. He then waits for a specific day to inject a treatment and harvest the sample. But, while the chick development process is rapid, well understood, and associated with a known timeline, it is not the only animal model system he uses. Other species that he has studied include spiders, crustaceans and mice. At different phases of treatment and development, there are always samples to dissect, slides to prepare with Vectastain® ABC for in situ hybridization or immunohistochemistry to assess protein expression, and other experiments to run. And there is always a lot of writing of manuscripts and abstracts, tucked into the incubation steps of protocols where hands-on work isn’t required. Sometimes he shares the most interesting images of his research with his fiancée, who not only likes the colors of the images, but also shares in Christian’s excitement about the knowledge they impart and the questions they answer.
“As a postdoc, you also spend a lot of time writing. Right now, I’m writing a manuscript and an abstract, so I have to find time for writing between each wash of the experiments that I’m doing. Sometimes my mentor sends me some articles to review because it helps her and it’s a good practice for me to work on a peer review. During the summer, we also have programs with high school students to give them the opportunity to learn what life in the lab is like and learn some benchwork. I also have the opportunity to supervise PhD students. I always have someone coming by to ask questions, which is always a good experience.”
Christian is attracted to the rigor and scope of academia and, in the long-term, sees himself splitting his time equally between research in his own lab and teaching. He hopes to elucidate the basic information underlying craniofacial phenotype development and ultimately find a way to modulate these craniofacial apparatus disorders. He believes the only way forward is to bridge the gaps that exist between the fields of medicine, pharmacology, and research in developmental biology and other areas, focusing less on simply answering the scientific questions and more on the production of novel translational treatments that can help children.
“We can use this mutant chick model to understand what’s wrong, try to correct some of the phenotypes seen in the model with a drug, and then use that drug in the future in humans. That potential is very exciting. Not everyone who works in craniofacial biology has the accessibility that this mutant provides to us. Most of the papers in this field finish by saying that we need to use these new insights to help the children and human health disorders. I agree wholeheartedly.”





Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.