Vector® TrueVIEW® Autofluorescence Quenching Kit
Dramatically reduce autofluorescence to reveal true immunofluorescence
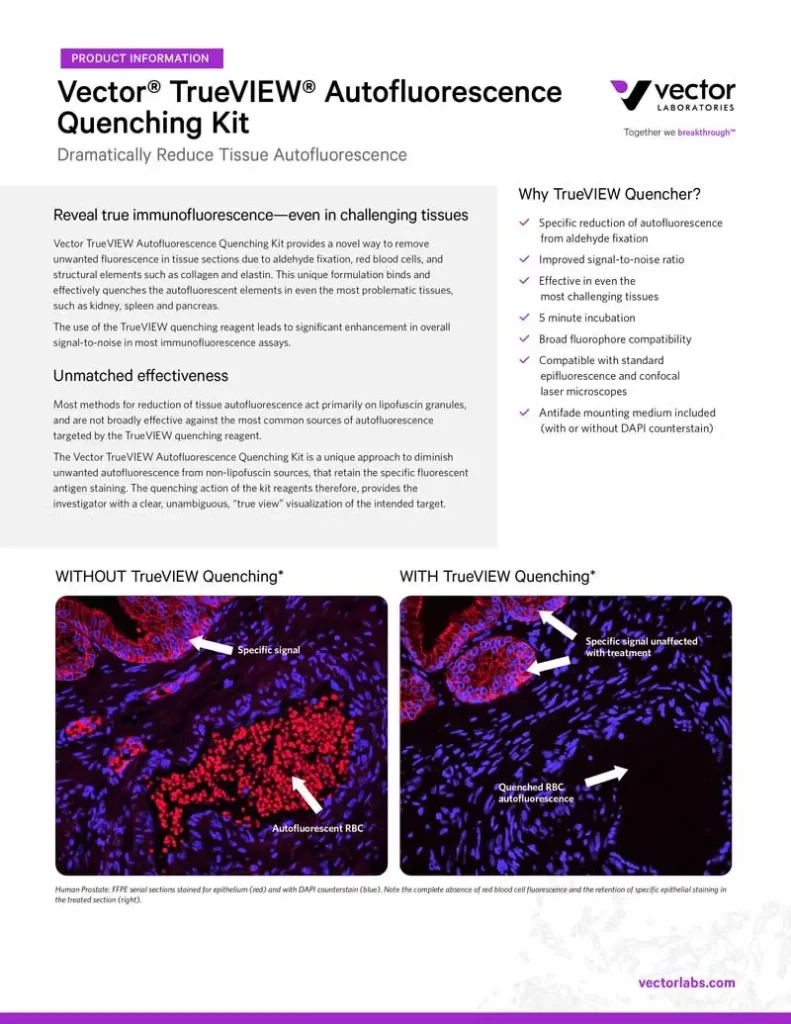
In tissue sections, autofluorescence is the unwanted fluorescence that can make it difficult or impossible to distinguish antigen-specific signal from non-specific background noise. The novel TrueVIEW Autofluorescence Quenching Kit specifically binds and quenches autofluorescent elements from non-lipofuscin sources, significantly enhancing signal-to-noise in most immunofluorescence assays—even in the most challenging tissues.
WITH TrueVIEW Quencher
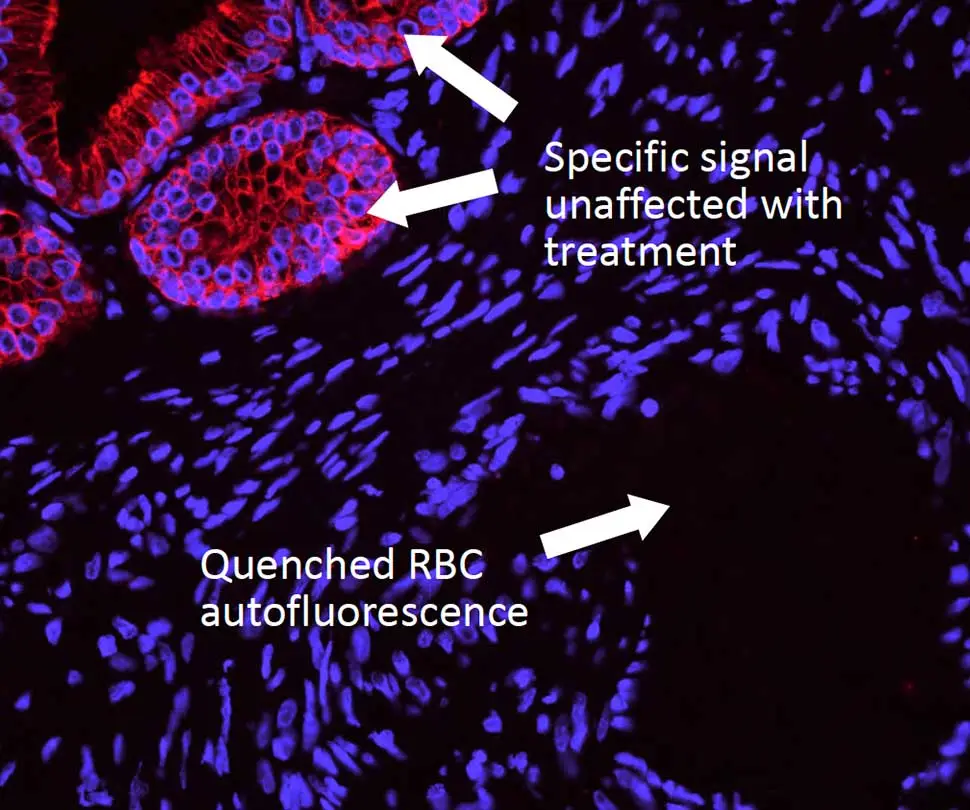
WITHOUT Treatment
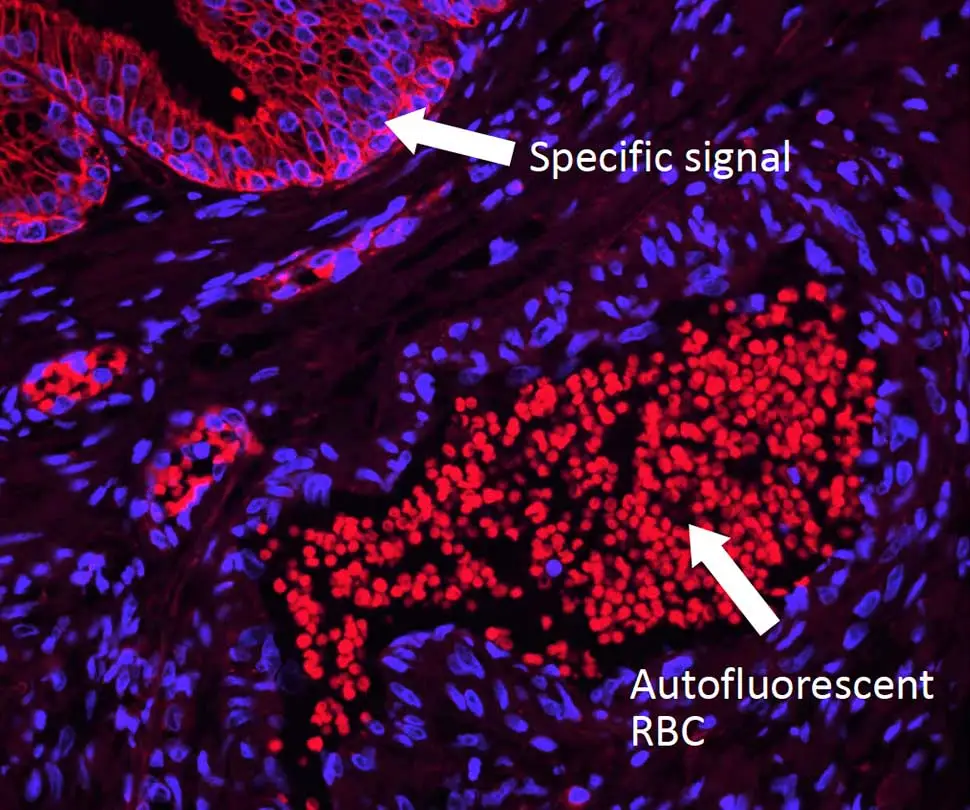
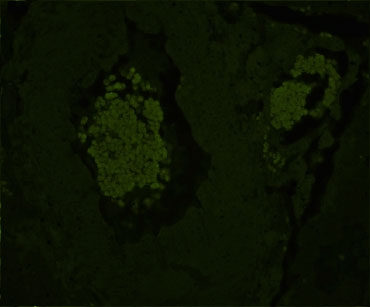
Reduction of autofluorescence in human spleen using the TrueVIEW Autofluorescence Quenching Kit. Human spleen sections (FFPE) stained using mouse anti-CD20 (red) and rabbit anti-Ki67 (green) primary antibodies detected with VectaFluor™ Duet kit (DK-8818). Note significant reduction of autofluorescence in the treated section.
Why TrueVIEW Quencher?
- Specific reduction of autofluorescence from non-lipofuscin sources
- Easy-to-use, one-step method
- Quick 5 min incubation
- Compatible with a wide selection of fluorophores
- Compatible with standard epifluorescence and confocal laser microscopes
Comparison with other autofluorescence reducing agents
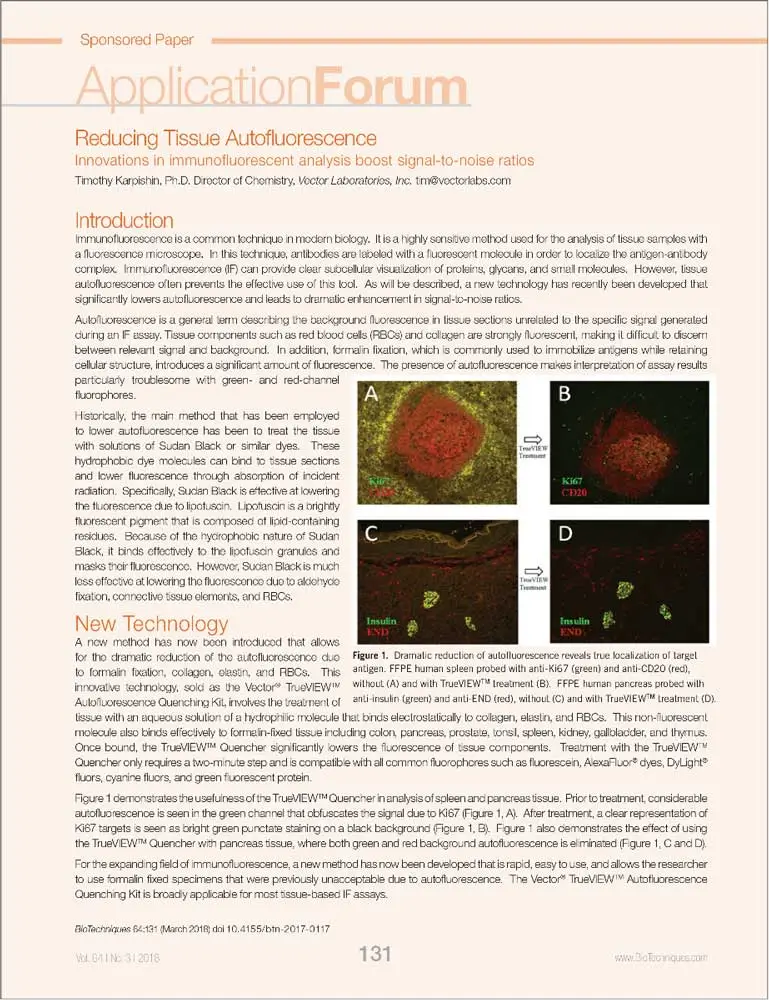
Whereas most methods for reducing tissue autofluorescence act primarily on lipofuscin granules, the TrueVIEW quencher targets fluorescence from non-lipofuscin sources, including aldehyde fixation, red blood cells, and structural elements, such as collagen and elastin. It provides a clear, unambiguous “true view” of target antigen localization, even in problematic tissues, such as kidney, spleen, and pancreas.
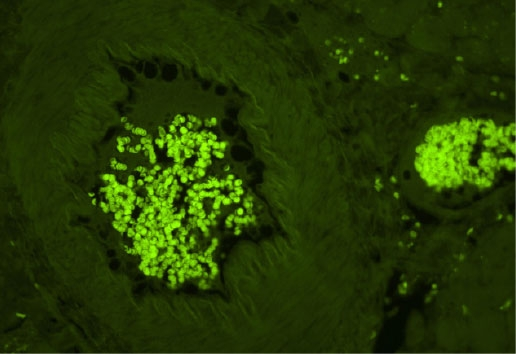
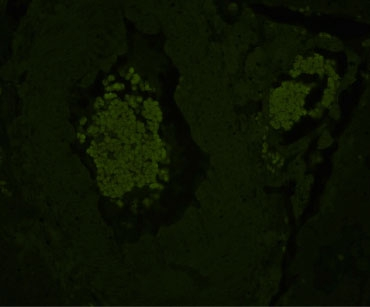
Comparisons with other commercial and “home brew” approaches show that Vector TrueVIEW is easier to use and more effective at reducing autofluorescence. The images below show the results of side-by-side comparisons on serial sections of formalin-fixed, paraffin embedded human pancreas visualized using a standard fluorescein (green) filter. No specific immunofluorescence staining was conducted.
No Treatment (Endogenous autofluorescence)
TrueVIEW Quencher Treated

Competitive treatments for dealing with autofluorescence
Company A
Company B
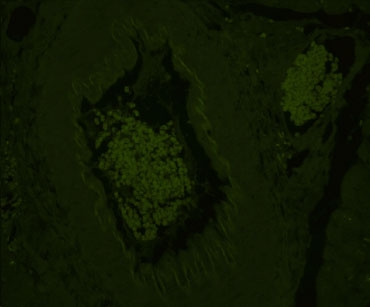
Company C
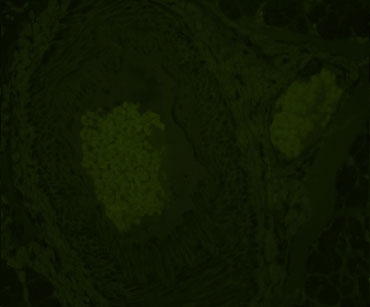
Alternative treatments for dealing with autofluorescence
Copper Sulfate Solution
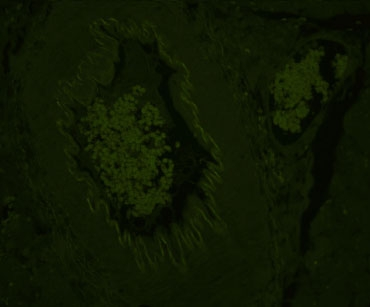
Sodium Borohydride
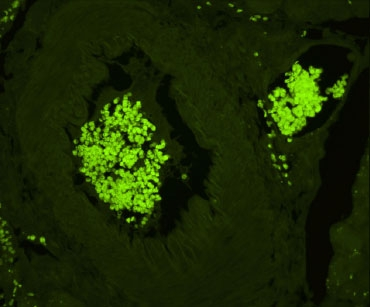
Sudan Black B
Additional resources available from Vector Labs
Listen to the podcast
LISTEN to the podcast by Timothy Karpishin, PhD, Director of Chemistry at Vector Laboratories, Inc. describing TrueVIEW Autofluorescence Quenching Kit at the 43rd Annual NSH Symposium/Convention.
Download the white paper
DOWNLOAD the white paper to learn about the issue of autofluorescence in immunofluorescence applications along with innovations that reduce autofluorescence and improve signal to noise ratios.
Download the brochure
Learn more about the Vector TrueVIEW Autofluorescence Quenching Kit by downloading the product brochure.
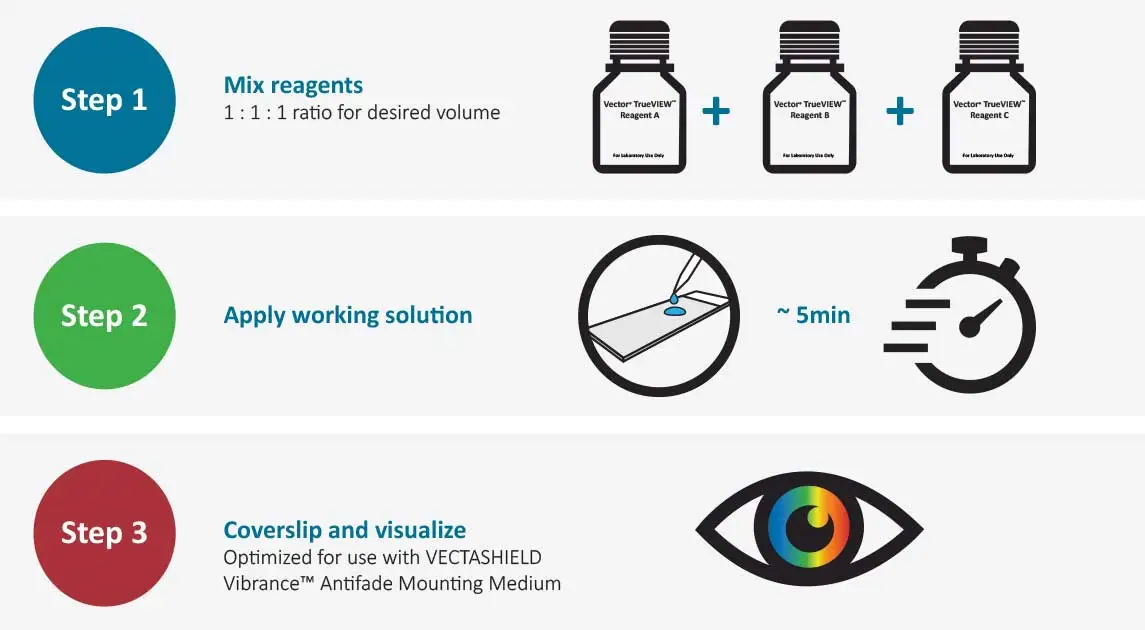
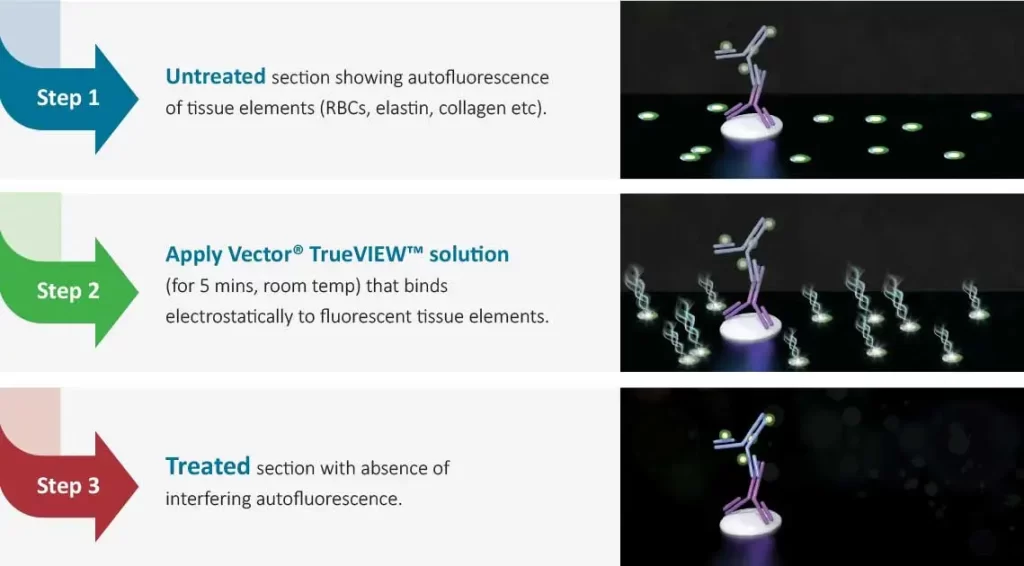
The TrueVIEW Autofluorescence Kit is easy to use
Following completion of immunofluorescence staining:
Mode of Action
Product Details & Ordering Information
| Product | Catalog Number | Applications | Unit Size |
|
TrueVIEW Autofluorescence Quenching Kit |
SP-8400 | Immunofluorescence, In situ hybridization | 15 mL |
|
TrueVIEW Autofluorescence Quenching Kit with DAPI |
SP-8500 | Immunofluorescence, In situ hybridization | 15 mL |
|
VECTASHIELD® Vibrance™ Antifade Mounting Medium |
H-1700 | Immunofluorescence, In situ hybridization, Cellular Imaging | 2 mL, 10 mL |
|
VECTASHIELD Vibrance™ with DAPI Antifade Mounting Medium |
H-1800 | Immunofluorescence, In situ hybridization, Cellular Imaging | 2 mL, 10 mL |
Publications:
Blanco, S. et al. 2020. Hyaluronate Nanoparticles as a Delivery System to Carry Neuroglobin to the Brain after Stroke. Pharmaceutics
Vinton, C.L. et al. 2019. Simian Immunodeficiency Virus Infection of Rhesus Macaques Results in Delayed Zika Virus Clearance. mBio
Oswald, D.M., Jones, M.B., Cobb, B.A. 2019. Modulation of hepatocyte sialylation drives spontaneous fatty liver disease and inflammation. Glycobiology
Ushioda, W. et al. 2019. >Neuropathology in Neonatal Mice After Experimental Coxsackievirus B2 Infection Using a Prototype Strain, Ohio-1. Journal of Neuropathology & Experimental Neurology
Bencze, J., et al. 2019. Neuropathological characterization of Lemur tyrosine kinase 2 (LMTK2) in Alzheimer’s disease and neocortical Lewy body disease. Scientific Reports
Davies, S.P. et al. 2019. Hepatocytes Delete Regulatory T Cells by Enclysis, a CD4+ T Cell Engulfment Process. Cell Reports
Liebers, J. et al. 2019. 3D image analysis reveals differences of CD30 positive cells and network formation in reactive and malignant human lymphoid tissue (classical Hodgkin Lymphoma). PLoS One
Lecocq, Q. et al. 2019. Noninvasive Imaging of the Immune Checkpoint LAG-3 Using Nanobodies, from Development to Pre-Clinical Use. Biomolecules
Motoike, S. et al. 2019. Clumps of Mesenchymal Stem Cell/Extracellular Matrix Complexes Generated with Xeno-Free Conditions Facilitate Bone Regeneration via Direct and Indirect Osteogenesis. International Journal of Molecular Sciences
Wilson, M.R. et al. 2019. ARID1A and PI3-kinase pathway mutations in the endometrium drive epithelial transdifferentiation and collective invasion. Nature Communications
Nagai-Okatani, C. et al. 2019. Wisteria floribunda agglutinin staining for the quantitative assessment of cardiac fibrogenic activity in a mouse model of dilated cardiomyopathy. Laboratory Investigation
Sheller-Miller, S. et al. 2019. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. American Journal of Obstetrics and Gynecology
Abe, H. et al. 2019. Correlation between platelet thrombus formation on collagen-coated beads and platelet aggregation induced by ADP. Transfusion and Apheresis Science
Singh, B. et al. 2019. Tau is required for progressive synaptic and memory deficits in a transgenic mouse model of α-synucleinopathy. Acta Neuropathologica
Chafe, S.C. et al. 2019. Targeting hypoxia-induced carbonic anhydrase IX enhances immune-checkpoint blockade locally and systemically. Cancer Immunol Res
Rhodes, S. et al. 2019. Cdkn2a (Arf) loss drives NF1-associated atypical neurofibroma and malignant transformation. Human Molecular Genetics
Rodgers, H.M. et al. 2019. Dopamine D1 and D3 receptor modulators restore morphine analgesia and prevent opioid preference in a model of neuropathic pain. Neuroscience
Yoon, J.H., Li, M., Basile, J.R., Lin, Y 2018. Computer-assisted analysis of immunohistological parameters in oral giant cell granulomas. Oral Diseases
Nishimura, A. et al. 2018. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission–associated myocardial senescence. Science Signaling
Su Y, Hou Y, Wang Q. 2018. The enhanced replication of an S-intact PEDV during coinfection with an S1 NTD-del PEDV in piglets. Veterinary Microbiology
Soontornniyomkij, V. et al. 2018. Association of antiretroviral therapy with brain aging changes among HIV-infected adults. AIDS
Du, H. et al. 2018. A novel mouse model of hemangiopericytoma due to loss of Tsc2. Human Molecular Genetics
Boucher, J.M. et al. 2018. Rab27a regulates human perivascular adipose progenitor cell differentiation. Cardiovascular Drugs and Therapy



