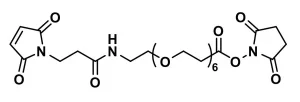
Description
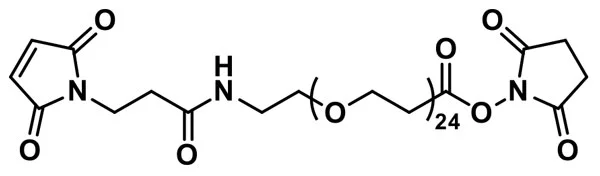
MAL-dPEG®24-NHS ester, product number QBD-10314, is a crosslinking reagent that joins a sulfhydryl to a free amine through a hydrophilic bridge. The sulfhydryl groups react with a maleimide group via a Michael addition reaction. The amines form amide bonds with the crosslinker by nucleophilic substitution of the N-hydroxysuccinimidyl (NHS) ester of a carboxylic acid group. The maleimide and NHS functional groups on the crosslinking compound sit at either end of a long, discrete-length polyethylene glycol chain (dPEG®).
Reactions that join free amines with free thiols are among the most popular, most useful crosslinking reactions in bioconjugate chemistry. These reactions require heterobifunctional reagents that bridge the two groups. Traditional crosslinkers are hydrophobic molecules. Vector Laboratories’ dPEG® crosslinking products are water-soluble, amphiphilic, single molecular weight PEG compounds with discrete chain lengths.
The conjugation of conventional hydrophobic crosslinking reagents to biomolecules almost inevitably triggers problems such as aggregation and precipitation of the conjugates. These problems do not occur with our water-soluble, non-immunogenic dPEG® crosslinkers.
Because NHS esters hydrolyze readily in water or aqueous buffer, the NHS ester end of the molecule must conjugate to a target molecule before conjugating the maleimide end of the molecule. At pH 7.0 – 7.5, NHS esters react optimally with free amines. However, NHS esters can react with free amines with pH as low as 6.5. As the pH increases, the hydrolysis rate of the NHS ester increases. Indeed, at pH 8 and 25°C, the half-life of an NHS ester in aqueous media is one hour, while at pH 8.6 and 4°C, the half-life falls to ten (10) minutes.[3] Thus, we strongly discourage storing MAL-dPEG®24-NHS ester, product number QBD-10314, in water or aqueous buffer. Instead, we recommend that customers make new solutions of the product as needed, use them immediately, and discard unused solutions after use.
The reaction of the maleimide end of MAL-dPEG®24-NHS ester with a sulfhydryl proceeds optimally at pH 6.5 – 7.5. Use the lowest reasonable pH within this range. Above pH 7.5, free amines compete with free thiols at the maleimide reaction site, which can cause confusing results. Moreover, at higher pH values, the maleimide ring may open to form unreactive maleamic acid.
The use of MAL-dPEG®24-NHS ester, product number QBD-10314, has been published in numerous scientific paper and patents. The following list highlights some of the more important uses of this product:
extending the in vivo half-life of a therapeutic diabody;
targeted delivery of a cancer drug via polylysine dendrimers;
targeted delivery of drugs to the lungs;
development of a blood purification device using magnetic nanoparticles;
development of a targeted therapeutic system using superparamagnetic nanoparticles;
improved transfection of tumor cells by PEGylated liposomes;
development of immunosensors and biosensors;
investigation of an aptamer using atomic force microscopy;
attaching antibodies to atomic force microscopy probes;
development of molecular pincers;
improvement of a peptidic ligand used for capture and purification of IgM; and,
surface coating of nanoparticles.
References
Greg T. Hermanson, Bioconjugate Techniques, 2nd Edition, Elsevier Inc., Burlington, MA 01803, April, 2008 (ISBN-13: 978-0-12-370501-3; ISBN-10: 0-12-370501-0); See pp. 276-335 for general description and use of heterobifunctional crosslinkers, as well as his specific discussion with protocols of our MAL-dPEG®x-NHS esters on pp. 718-722.
Greg T. Hermanson, Bioconjugate Techniques, 3rd Edition, Elsevier, Waltham, MA 02451, 2013, ISBN 978-0-12-382239-0; See Chapter 18, Discrete PEG Reagents, pp. 787-821, for a full overview of the dPEG® products.
Antigen Peptide-Based Immunosensors for Rapid Detection of Antibodies and Antigens. Ling Tian and Tomasz Heyduk. Analytical Chemistry. 2009, 81 (13) pp 5218–5225. May 26, 2009. DOI: 10.1021/ac900845a.
Characterization and tumour targeting of PEGylated polylysine dendrimers bearing doxorubicin via a pH labile linker. Lisa M. Kaminskas, Brian D. Kelly, Victoria M. McLeod, Gian Sberna, David J. Owen, Ben J. Boyd, Christopher J.H. Porter. Journal of Controlled Release. 2011, 152 (2) Pages 241-248. June 10, 2011. DOI: 10.1016/j.jconrel.2011.02.005.
Device for continuous extracorporeal blood purification using target-specific metal nanomagnets. Inge K. Herrmann, Riccardo E. Bernabei, Martin Urner, Robert N. Grass, Beatrice Beck-Schimmer and Wendelin J. Stark. Nephrol Dial Transplant. 2011, 26 (9) pp 2948-2954. February 10, 2011. DOI: 10.1093/ndt/gfq846.
Functional improvement of an IRQ-PEG-MEND for delivering genes to the Lung. Taichi Ishitsuka, Hidetaka Akita, Hideyoshi Harashima. Journal of Controlled Release. 2011, 154 (1) pp 77-83, August 25, 2011. DOI: 10.1016/j.jconrel.2011.05.012.
Interactive Configuration through Force Analysis of GM1 Pentasaccharide-Vibrio cholera Toxin Interaction. Jeong Hyun Seo, Chang Sup Kim, Hea Yeon Lee, Tomoji Kawai, and Hyung Joon Cha. Analytical Chemistry. 2011, 83 (15) pp 6011–6017. June 24, 2011. DOI: 10.1021/ac201013p.
Cyclic and dimeric gluten peptide analogues inhibiting DQ2-mediated antigen presentation in celiac disease. Jiang Xia, Elin Bergseng, Burkhard Fleckenstein, Matthew Siegel, Chu-Young Kim, Chaitan Khosla and Ludvig M. Sollid. Bioorganic & Medicinal Chemistry. 2007, 15 (20), pp 6565–6573. October 15, 2007. DOI:10.1016/j.bmc.2007.07.001.
Efficient capture of circulating tumor cells with a novel immunocytochemical microfluidic device. Mary Nora Dickson, Pavel Tsinberg, Zhongliang Tang, Farideh Z. Bischoff, Timothy Wilson, and Edward F. Leonard. Biomicrofluidics. 2011, 5 (3). August 22, 2011. DOI: 10.1063/1.3623748.
A methodology for preparing nanostructured biomolecular interfaces with high enzymatic activity. Lu Shin Wong, Chinnan V. Karthikeyan, Daniel J. Eichelsdoerfer, Jason Micklefield and Chad A. Mirkin. Nanoscale. 2012, 4 (2) pp 659-666. December 8, 2011. DOI:10.1039/C1NR11443C.
Investigation of the Interaction between a Bivalent Aptamer and Thrombin by AFM. Lin Ge, Gang Jin and Xiaohong Fang. Langmuir. 2012, 28 (1) pp 707–713. November 21, 2011. DOI: 10.1021/la203954x.
Immobilization of hydrophobic peptidic ligands to hydrophilic chromatographic matrix: A preconcentration approach. Satyen Gautam, Kai-Chee Loh. Analytical Biochemistry. 2012, 423 (2) pp 202-209. April 15, 2012. DOI: 10.1016/j.ab.2012.01.020.
Targeting of Primary Breast Cancers and Metastases in a Transgenic Mouse Model Using Rationally Designed Multifunctional SPIONs. Forrest M. Kievit, Zachary R. Stephen, Omid Veiseh, Hamed Arami, Tingzhong Wang, Vy P. Lai, James O. Park, Richard G. Ellenbogen, Mary L. Disis, and Miqin Zhang. ACS Nano. 2012, 6 (3) pp 2591–2601. February 10, 2012. DOI: 10.1021/nn205070h.
Subcellular trafficking and transfection efficacy of polyethylenimine-polyethylene glycol polyplex nanoparticles with a ligand to melanocortin receptor-1. Mikhail O. Durymanov, Elena A. Beletkaia, Alexey V. Ulasov, Yuri V. Khramtsov, Georgiy A. Trusov, Nikita S. Rodichenko, Tatiana A. Slastnikova, Tatiana V. Vinogradova, Natalia Y. Uspenskaya, Eugene P. Kopantsev, Andrey A. Rosenkranz, Eugene D. Sverdlov, Alexander S. Sobolev. Journal of Controlled Release. 2012, 163 (2) pp 211-219. October 28, 2012. DOI: 10.1016/j.jconrel.2012.08.027.
Antibody-Unfolding and Metastable-State Binding in Force Spectroscopy and Recognition Imaging. Parminder Kaur, Qiang-Fu, Alexander Fuhrmann, Robert Ros, Linda Obenauer Kutner, Lumelle A. Schneeweis, Ryman Navoa,Kirby Steger, Lei Xie, Christopher Yonan, Ralph Abraham, Michael J. Grace, and Stuart Lindsay. Biophysical Journal. 2011, 100 (1) pp 243-250. January 5, 2011. DOI:10.1016/j.bpj.2010.11.050.
Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified pegylated liposomes. Amit A. Kale and Vladimir P. Torchilin. Journal of Drug Targeting. 2007, 15 (7-8) pp 538-545. September 13, 2007. DOI:10.1080/10611860701498203.
Multifunctional nanoagent for thrombus-targeted fibrinolytic Therapy. Jason R. McCarthy, Irina Y. Sazonova, S. Sibel Erdem, Tetsuya Hara, Brian D. Thompson, Purvish Patel, Ion Botnaru, Charles P. Lin, Guy L. Reed, Ralph Weissleder, and Farouc A. Jaffer. Nanomedicine 2012 7 (7) pp 1017–1028. February 21, 2012. DOI:10.2217/nnm.11.179.
Properties of PEI-based Polyplex Nanoparticles That Correlate With Their Transfection Efficacy. Alexey V Ulasov, Yuri V Khramtsov, Georgiy A Trusov, Andrey A Rosenkranz, Eugene D Sverdlov and Alexander S Sobolev. Molecular Therapy. 2011, 19 (1) pp 103-112. November 2, 2010. DOI:10.1038/mt.2010.233.
Genetic PEGylation. Seiichi Tada, Takashi Andou, Takehiro Suzuki, Naoshi Dohmae, Eiry Kobatake and Yoshihiro Ito. PLoS One. 2012, 7 (11) pp e49235. November 8, 2012. DOI:10.1371/journal.pone.0049235.
Human pIgR mimetic peptidic ligand for affinity purification of IgM Part II: Ligand binding characteristics. Satyen Gautam, Kai-Chee Loh. Separation and Purification Technology. 2013, 102 pp 43–49. January 4, 2013. DOI: 10.1016/j.seppur.2012.09.024.
Attaching Antibodies to AFM Probes with the Sulfhydryl Reactive PEG Tether, NHS-PEG18-PDP. W. Travis Johnson, Ph.D. Agilent Technologies, Inc. 2007, 5989-7702EN. December 18, 2007.
Biomimetic Ligands for Immunoglobulin-M Purification. Satyen Gautam. Doctoral Dissertation, National University of Singapore: Singapore, April 12, 2010. DOI: www.scholarbank.nus.edu.sg/handle/10635/22872.
Development of a novel bead-based96-well filtrationplate competitive immunoassay for the detection of Gentamycin. Tien Yu Jessica Ho, Chia-ChungChan, KingHoChan, YuChiehWang, Jing-TangLin, Cheng-Ming Chang, Chien-ShengChen. Biosensors and Bioelectronics. 2013, 49 pp 126–132. November 15,2013. DOI: 10.1016/j.bios.2013.04.027.
MicroSPECT/CT imaging of co-expressed HER2 and EGFR on subcutaneous human tumor xenografts in athymic mice using 111In-labeled bispecific radioimmunoconjugates. Eva Razumienko, Lindsay Dryden,
Deborah Scollard, Raymond M. Reilly. Breast Cancer Research and Treatment. 2013, 138 (3) pp 709-718. March 24, 2013. DOI: 10.1007/s10549-013-2490-5.
Small-Animal SPECT/CT of HER2 and HER3 Expression in Tumor Xenografts in Athymic Mice Using Trastuzumab Fab–Heregulin Bispecific Radioimmunoconjugates. Eva J. Razumienko, Deborah A. Scollard, and Raymond M. Reilly. Journal of Nuclear Medicine. 2012, 53 (12) pp 1943–1950. October 24, 2012. DOI: 10.2967/jnumed.112.106906.
Delivery of Small Interfering RNA by Peptide-Targeted Mesoporous Silica Nanoparticle-Supported Lipid Bilayers. Carlee E. Ashley, Eric C. Carnes, Katharine E. Epler, David P. Padilla, Genevieve K. Phillips, Robert E. Castillo, Dan C. Wilkinson, Brian S. Wilkinson, Cameron A. Burgard, Robin M. Kalinich, Jason L. Townson, Bryce Chackerian, Cheryl L. Willman, David S. Peabody, Walker Wharton and C. Jeffrey Brinker. ACS Nano. 2012, 6 (3) pp 2174-2188. February 6, 2012. DOI: 10.1021/nn204102q.
BMP-2 tethered hydroxyapatite for bone tissue regeneration: Coating chemistry and osteoblast attachment. Stefanie M. Shiels, Kimberly D. Solomon, Marcello Pilia,Mark R. Appleford, Joo L. Ong. Journal of Biomedical Materials Research. 2012, 100A (11) pp 3117-3123. July 20, 2012. DOI: 10.1002/jbm.a.34241.
Extracellular Antibody Drug Conjugates Exploiting the Proximity of Two Proteins. David J Marshall, Scott S Harried, John L Murphy, Chad A Hall, Mohammed S Shekhani, Christophe Pain, Conner A Lyons, Antonella Chillemi, Fabio Malavsi, Homer L Pearce, Jon S Thorson, and James R Prudent. MT Open. 2016. July 19, 2016. DOI: 10.1038/mt.2016.119.
A study of polyethylene glycol backfilling for enhancing target recognition using QCM-D and DPI. Yanqiu Du, Jing Jin, and Wei Jiang. Journal of Materials Chemistry B. 2018, 6, pp 6217-6224. September 21, 2018. DOI: 10.1039/c8tb01526k.
Protein purification to analyze AAA+ proteolytic machine in vitro. Diego Rojas. Columbia University Academic Commons. 2011.
Nanomechanics of HaloTag Tethers. Ionel Popa, Ronen Berkovich, Jorge Alegre-Cebollada, Carmen L. Badilla, Jaime Andrés Rivas-Pardo, Yukinori Taniguchi, Masaru Kawakami, and Julio M. Fernandez. Journal of the American Chemical Society. 2013, 135 (34) pp 12762–12771. August 2, 2013. DOI: 10.1021/ja4056382.
SNAP-Tag Technology Mediates Site Specific Conjugation of Antibody Fragments with a Photosensitizer and Improves Target Specific Phototoxicity in Tumor Cells. Ahmad Fawzi Hussain, Florian Kampmeier, Verena von Felbert, Hans-F. Merk, Mehmet Kemal Tur, and Stefan Barth. Bioconjugate Chemistry. 2011, 22 (12) pp 2487–2495. October 13, 2011. DOI: 10.1021/bc200304k.
Application of HaloTag Protein to Covalent Immobilization of Recombinant Proteins for Single Molecule Force Spectroscopy. Yukinori Taniguchi and Masaru Kawakami. Langmuir. 2010, 26 (13) pp 10433–10436. June 9, 2010. DOI: 10.1021/la101658a.
Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Matthias T. Stephan, Sirkka B. Stephan, Peter Bak, Jianzhu Chen, Darrell J. Irvine. Biomaterials. 2012, 33 (23) pp 5776-5787. August 2012. DOI: 10.1016/j.biomaterials.2012.04.029.
Photodynamic characterization and optimization using multifunctional nanoparticles for brain cancer treatment. Kristen Herrmann, Yong-Eun Lee Koo, Daniel A. Orringer, Oren Sagher, Martin Philbert, Raoul Kopelman. SPIE Proceedings. 2013, 8568 (1) pp 1-8. March 13, 2013. DOI: 10.1117/12.2005530.
“Smart” Drug Carriers: PEGylated TATp-Modified pH-Sensitive Liposomes. Amit A. Kale and Vladimir P. Torchilin. Journal of Liposome Research. 2007, 17(3-4) pp 197-203. June 6, 2007. DOI: 10.1080/08982100701525035.
Therapeutic Anti-Methamphetamine Antibody Fragment-Nanoparticle Conjugates: Synthesis and in Vitro Characterization. Nisha Nanaware-Kharade , Guillermo A. Gonzalez , III, Jackson O. Lay , Jr., Howard P. Hendrickson , and Eric C. Peterson. Bioconjugate Chemistry. 2012, 23 (9) pp 1864–1872. August 9, 2012. DOI: 10.1021/bc300204n.
Molecular Pincers: Antibody-Based Homogeneous Protein Sensors. Ewa Heyduk, Benjamin Dummit, Yie-Hwa Chang, and Tomasz Heyduk. Analytical Chemistry. 2008, 80 (13) pp 5152-5159. May 21, 2008. DOI: 10.1021/ac8004154.
Specific Binding at the Cellulose Binding Module-Cellulose Interface Observed By Force Spectroscopy. Jason R King, Carleen M. Bowers and Eric J Toone. Langmuir. 2015, 31 (11) March 4, 2015. DOI: 10.1021/la504836u.
A Nanotechnology-Based Platform for Extending the Pharmacokinetic and Binding Properties of Anti-methamphetamine Antibody Fragments. Nisha Nanaware-Kharade, shraddha Thakkar, Guillermo A. Gonzalez III, and Eric C. Peterson. Scientific Reports. 2014, 5 (12060). July 10, 2015. DOI: 10.1038/srep12060.
Serum albumin ‘camouflage’ of plant virus based nanoparticles prevents their antibody recognition and enhances pharmacokinetics. Andrzej S. Pitek, Slater A. Jameson, Frank A. Veliz, Sourabh Shukla, and Nicole F. Steinmetz. Biomaterials. 2016, 89 pp 89-97, February 23, 2016. DOI: 10.1016/j.biomaterials.2016.02.032.
Optimization, Production and Characterization of a CpG-Oligonucleotide-Ficoll Conjugate Nanoparticle Adjuvant for Enhanced Immunogenicity of Anthrax Protective Antigen. Bob Milley, Radwan Kiwan, Gary S. Ott, Carlo Calacsan, Melissa Kachura, John D Campbell, Holger Kanzler, and Robert L. Coffman. Bioconjugate Chemistry. 2016, April 13, 2016. DOI: 10.1021/acs.bioconjchem.6b00107.
Application of vasoactive and matrix-modifying drugs can improve polypex delivery to tumors upon intravenous administration. Mikhail O. Durymanov, Alexey V. Yarutkin, Dmitry V. Bagrov, Dmitry V. Klinov, Alexander V. Kedrov, Nikolay K. Chemeris, Andrey A. Rosenkranz, and Alexander S. Sobolev. Journal of Controlled Release. 2016, 232 pp 20-28. April 6, 2016. DOI: 10.1016/j.jconrel.2016.04.011.
Surface Force Analysis of Pyrite (FeS2): Its Reactivity to Amino Acid Adsorption. Narangerel Ganbaatar, Nina Matsuzaki, Yuya Nakazawa, Rehana Afrin, Masashi Aono, Taka-aki Yano, Tomohiro Hayashi, and Masahiko Hara. Advances in Materials Physics and Chemistry. 2016, 6, pp 167-176. June 30, 2016. http://dx.doi.org/10.4236/ampc.2016.67018.
Immune Response Modulation of Conjugated Agonists with Changing Linker Length. Keun Ah Ryu, Katarzyna Slowinska, Troy Moore, and Aaron Esser-Kahn. ACS Chemical Biology. 2016, October 17, 2016. DOI: 10.1021/acschembio.6b00895.
Tumor tissue slice cultures as a platform for analyzing tissue-penetration and biological activities of nanoparticles. Lea Merz, Sabrina Hobel, Sonja Kallendrusch, Alexander Ewe, Ingo Bechmann, Heike Franke, Felicitas Merz, and Achim Aigner. Science Direct. 2016, 112, pp 45-50. http://dx.doi.org/10.1016/j.ejpb.2016.11.0163.
Optimized polyethylenimine (PEI)-based nanoparticles for siRNA delivery, analyzed in vitro and in an ex vivo tumor tissue slice culture model. Alexander Ewe, Sabrina Hobel, Claudia Heine, Lea Merz, Sonja Kallendrusch, Ingo Bechmann, Felicitas Merz, Heike Franke, Achim Aigner. Drug Delivery and Translational Research. 2016, pp 1-11. June 22, 2016. DOI: 10.1007/s13346-016-0306-y.
Development of Raman Spectroscopy as a clinical Diagnostic Tool. Santa Borel. Doctoral Disseratation, University of Toronto, Toronto, Ontario, Canada. 2016, pp 1-99. November 2016. https://tspace.library.utoronto.ca/handle/1807/74538
Mapping of Molecular Structure of the Nanoscale Surface in Bio-nanoparticles. Luciana M Herda, Delyan R Hristov, Maria Cristina Lo Giudice, Ester Polo, and Kenneth A Dawson. Journal of the American Chemical Society. 2016. December 22, 2016. DOI: 10.1021/jacs.6b12297.
64Cu-Labeled Trastuzumab Fab-PEG24-EGF Radioimmunoconjugates Bispecific for HER2 and EGFR-Pharmacokinetics, Biodistribution, and Tumor Imaging by PET in Comparison to Monospecific Agents. Luke (Yongkyu) Kwon, Deborah A Scollard, and Raymond M Reilly. Molecular Pharmaceutics. 2017, pp 1-38. January 3, 2017. DOI: 10.1021/acs.molpharmaceut.6b00963.
Quantitative Evaluation of Viral Protein Binding to Phosphoinositide Receptors and Pharmacological Inhibition. Seong-Oh Kim, Joshua A. Jackman, Menashe Elazar, Sang-Joon Cho, Jeffrey S Glenn, and Nam-Joon Cho. Analytical Chemistry. 2017, August 15, 2017. DOI: 10.1021/acs.analchem.7b01568.
Silica Nanoparticles for the Delivery of DNA and RNAi in Cancer Treatment. Michael Aaron Vrolijk. Graduate College Dissertations and Theses. 2017, pp 1-122. May 22, 2017. http://scholarworks.uvm.edu/graddis/783.
Inhibition of Myeloperoxidase Activity in Cystic Fibrosis Sputum by Peptide Inhibitor of Complement C1 (PIC1). Pamela S. Hair, Laura A. Sass, Neel K. Krishna, and Kenji M. Cunnion. PLOS one. 2017, 12 (1) e0170203. January 30, 2017. DOI: 10.1371/journal.pone.0170203.
Elongated plant virus-based nanoparticles for enhanced delivery of thrombolytic therapies. Andrzej Stanislaw Pitek, Yunmei Wang, Sahil Gulati, Huiyun Gao, Phoebe L Stewart, Daniel I. Simon, and Nicole F Steinmetz. Molecular Pharmaceutics. 2017, pp 1-9. September 7, 2017. DOI: 10.1021/acs.molpharmaceut.7b00559.
Evaluation of non-targeting, C- or N-pH (low) insertion peptide modified superparamagnetic iron oxide nanoclusters for selective MRI of liver tumors and their potential toxicity in cirrhosis. Abdulrahman Ahmed Mahmood, Jianqi Zhang, Rufang Liao, Xiwei Pan, Dan Xu, Haibo Xu, Qibing Zhou. RSC Advances. 2019, 9, 14051-14059. May 7, 2019. DOI: 10.1039/C9RA02430A.
Hydrogel Skin-Covered Neurons Self-Assembled with Gustatory Cells for Selective Taste Stimulation. Trang Huyen Le-Kim, Bon Il Koo, Jun Su Yun, Seung-Woo Cho, Yoon Sung Nam. ACS Omega. 2019, 4, 7, pp 12393-12401. July 19, 2019. DOI: 10.1021/acsomega.9b00998
Mesoporous Silica Nanoparticles as pH-Responsive Carrier for the Immune-Activating Drug Resiquimod Enhance the Local Immune Response in Mice. Julia Wagner, Dorothée Gößl, Natasha Ustyanovska, Mengyao Xiong, Daniel Hauser, Olga Zhuzhgova, Sandra Hočevar, Betül Taskoparan, Laura Poller, Stefan Datz, Hanna Engelke, Youssef Daali, Thomas Bein, and Carole Bourquin. ACS Nano. 2021. 2021, 15, 3, 4450–4466. 03/01/2021. DOI: 10.1021/acsnano.0c08384.
Applications of Atomic Force Microscopy and Nanopore Translocation in Nanoscale Microbial Surface and Single Molecules Studies. Jiawei Liu. Arizona State University ProQuest Dissertations. 2022. May 2022.
Investigations into the Ice Crystallization and Freezing Properties of the Antifreeze Protein ApAFP752. Asenath-Smith, Emily Jeng, Emily C. Ambrogi, Emma K. Hoch, Garrett R. Olivier, Jason L., Engineer Research and Development Center (U.S.), ERDC/CRREL TR-22-17, 09/01/2022, 10.21079/11681/45620