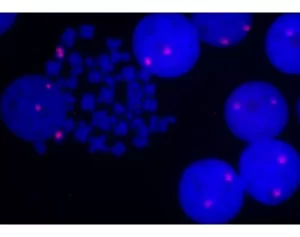
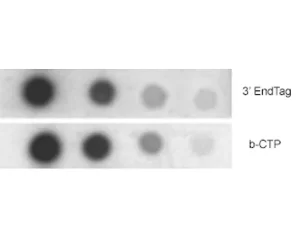
Description
The ChromaLINK Digoxigenin One-Shot Antibody Labeling Kit provides convenient, consistent, and measurable digoxigenin labeling of 100 µg of antibody. Each kit contains ChromaLINK Digoxigenin, which incorporates a novel UV-traceable chromophore in the linker arm to enable reproducibility in your antibody labeling process. With a simple, non-destructive UV scan you can now quantify digoxigenin incorporation to ensure reproducible incorporation of the optimal number of haptens per antibody. Each ChromaLINK One-Shot kit contains everything needed to to label your antibody: buffers, reagents, desalting columns, and an easy-to-follow protocol and online DIG incorporation calculator.
Technical Information
Product Description
The ChromaLINK Digoxigenin One-Shot antibody labeling kit is specifically designed to incorporate a digoxigenin label into a single 100 microgram quantity of antibody resuspended in 100 μl of buffer. This kit comes complete with all the necessary components to label and rapidly quantify incorporated digoxigenin from a small quantity of antibody in about 90 minutes.
Digoxigenin has long been used as an alternative hapten reporter system to the streptavidin/biotin system (1). This system is based on a steroid isolated from the blossoms and leaves of the plant Digitalis purpurea, the only known natural source of digoxigenin. High affinity antibodies raised against digoxigenin thus exhibit virtually no cross-reaction with other biological molecules found in higher organisms. Both the specificity and sensitivity of this “parallel” hapten-reporter system often gives digoxigenin a major advantage over biotin-based detection systems. In more recent times, this reporter system has also been used in conjunction with the biotin-streptavidin system to detect multiplexed antigens (2). Unlike the biotin/streptavidin system, the digoxigenin label has never had a method for directly quantifying incorporation, until now.
The labeling process used in the ChromaLINK Digoxigenin One-Shot kit is based on a patented UV-traceable linker called Sulfo-ChromaLINK Digoxigenin (Figure 1). This advanced digoxigenin labeling reagent contains an aromatic water-soluble N-hydroxy-sulfosuccinimidyl ester functional group (a), which efficiently modifies protein lysine residues in a phosphate buffer system. The linker also possesses an embedded bis-aryl hydrazone structure (b), which forms the compound’s UV-traceable chromophore. This traceable signature enables the non-destructive and direct quantification of digoxigenin (c), attached to the antibody. These diverse chemical elements are linked together through a long-chain PEG3 spacer (d), which preserves antibody-digoxigenin binding affinity while simultaneously maintaining antibody solubility.
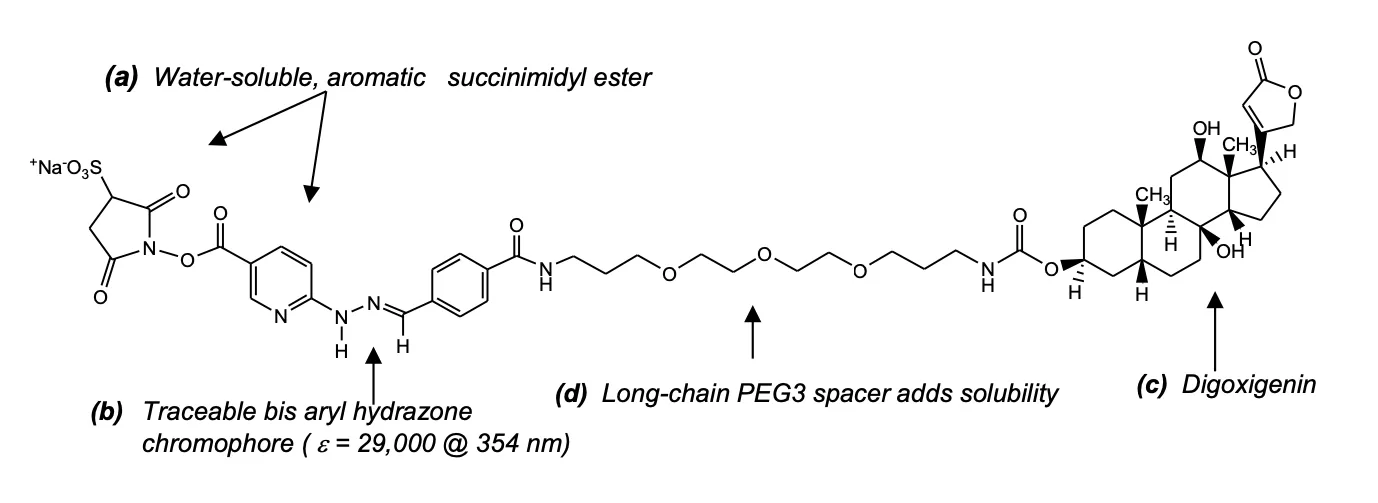 Figure 1: Structure of ChromaLINK Digoxigenin C52H67N6NaO17S; M.W. 1103.17
Figure 1: Structure of ChromaLINK Digoxigenin C52H67N6NaO17S; M.W. 1103.17
Benefits and Features
The ChromaLINK Digoxigenin One-Shot kit is a convenient, cost-effective way of incorporating digoxigenin into a small quantity (100 μg) of an antibody. After incorporation, digoxigenin is rapidly quantified using a non-destructive UV-scan (220-400 nm). The kit features high antibody recoveries (50-80%) with consistent digoxigenin incorporation. The degree of incorporation or molar substitution ratio (MSR) ranges from 2-8 digoxigenin molecules per antibody when used as directed.
The ChromaLINK Digoxigenin One-Shot kit can be used to label a diverse group of antibodies including all mammalian IgG, IgE, and avian IgY molecules. The ChromaLINK Digoxigenin One-Shot kit offers a robust method of both labeling and quantifying incorporated digoxigenin with a simple protocol. This “double-feature” provides researchers with greater confidence, consistency and reproducibility in all downstream applications.
Procedure Diagram
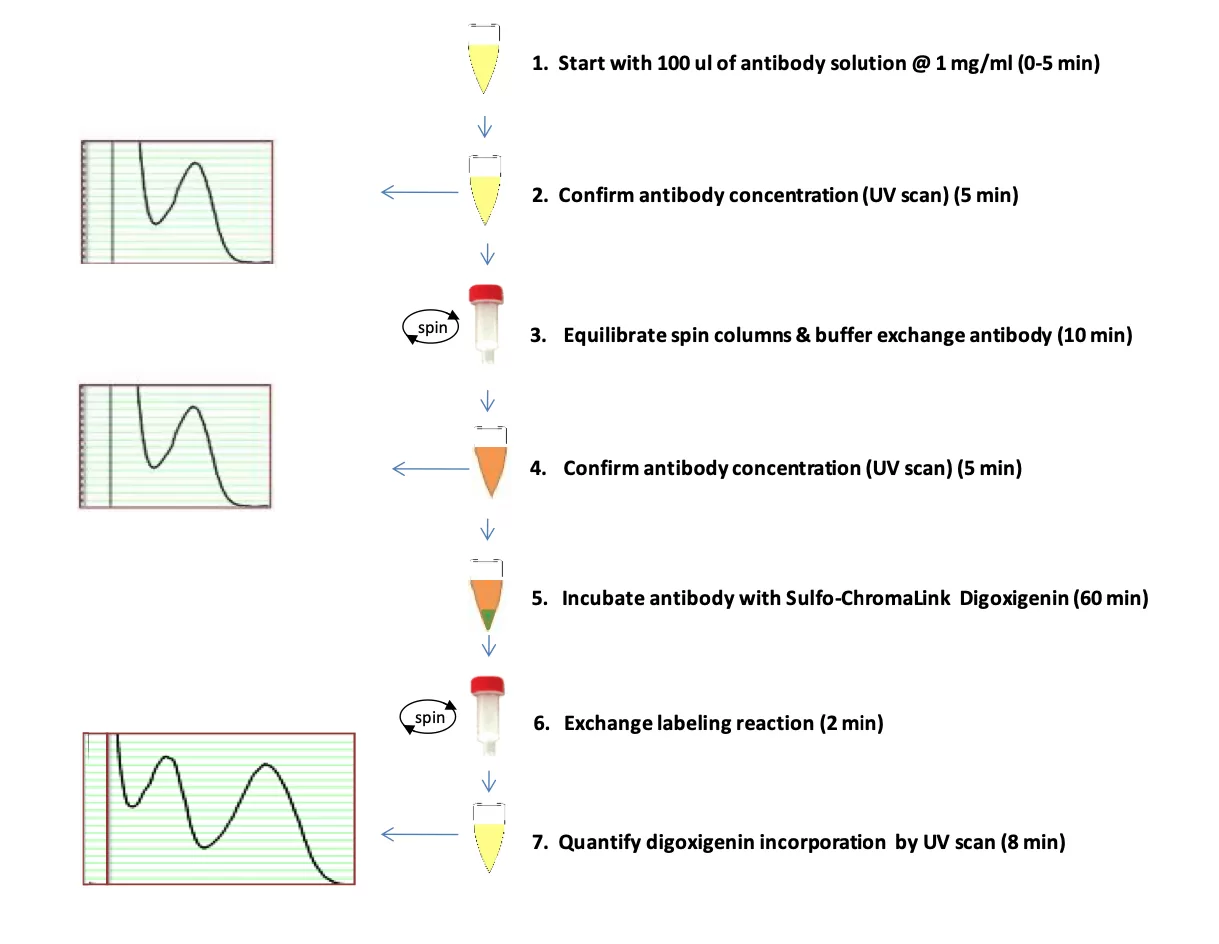
Figure 2. Illustration of the ChromaLINK Digoxigenin One-Shot antibody labeling procedure.
Process Summary
Sample Preparation: adjust antibody to 1 mg/ml in 100 μl buffer
Sample Analysis: confirm antibody concentration using a spectrophotometer
First Buffer Exchange: equilibrate spin columns & buffer exchange antibody
Sample Analysis: reconfirm recovered antibody concentration on a spectrophotometer
Digoxigenin Labeling: label antibody with ChromaLINK Digoxigenin linker
Second Buffer Exchange: remove excess labeling reagent with spin column
Digoxigenin Incorporation: quantify incorporation using a spectrophotometer
Important Labeling Parameters
The ChromaLINK Digoxigenin One-Shot antibody labeling kit is specifically designed to incorporate digoxigenin into a single 100 microgram quantity of antibody resuspended in 100 μl of a suitable buffer as indicated in Table 1.
Table 1. Initial starting conditions required for the ChromaLINK Digoxigenin One-Shot procedure.
The kit provides consistent and reliable incorporation of digoxigenin by controlling the following reaction variables:
- Initial antibody mass (100 μg) and volume (100 μl)
- Reaction buffer composition
- Reaction time (60 min)
- Reaction stoichiometry (15-fold mole-equivalents of linker)
Critical to digoxigenin incorporation is the ability to accurately determine an antibody’s initial concentration in a non-destructive manner with full recovery of the sample. The ChromaLINK Digoxigenin One-Shot procedure employs a spectral scan (220-400 nm) rather than a single wavelength measurement @ 280 nm to accomplish this task. Commercial antibodies often contain preservatives or other additives capable of masking or distorting the intrinsic UV spectrum of a sample. As a consequence, additives make it more difficult to accurately estimate protein concentration using a single wavelength (i.e. A280) measurement. A scan however, provides greater information and assurance that the antibody’s concentration is correct since a spectrum often reveals the presence of interfering additives.
Commonly used additives may include preservatives such as sodium azide, thimerosal, protein stabilizers such as *BSA or *gelatin, or small molecule additives such as glycine. *Note-protein carriers such as BSA or gelatin must be removed before labeling can proceed.


 Figure 1: Structure of ChromaLINK Digoxigenin C52H67N6NaO17S; M.W. 1103.17
Figure 1: Structure of ChromaLINK Digoxigenin C52H67N6NaO17S; M.W. 1103.17