Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
End labeling is a favored method for applications in which an internal label might interfere with hybridization or sequence specific protein binding.
| Unit Size | 1 kit |
|---|---|
| Target for Labeling | DNA, Oligonucleotides, RNA |
| Required Reactive Group | 5 ‘-OH group of nucleic acid |
| Tag/Group Incorporated | Thiol |
| Safety Title | WARNING: NA – www.P65Warnings.ca.gov |
Materials suitable to perform 10 end labeling reactions of up to 0.6 nmols of 5′ ends (e.g. about 5 μg of a 25 base oligo) per reaction. This includes:
*Note: Thiol-reactive labeling reagent is not included in the kit and must be purchased separately. Labels suitable for this kit include:
Note regarding shipping: This item is shipped on wet ice and therefore requires additional shipping charges.
End labeling is a favored method for applications in which the presence of an internal label might interfere with hybridization or sequence specific protein binding.
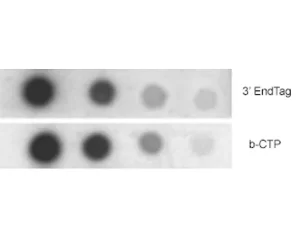
5′ or 3′ EndTag labeled nucleic acids can be used for applications such as DNA hybridization, PCR, in situ hybridization, the binding of capture probes to affinity matrices, or electrophoretic mobility shift assays (EMSA). Short oligonucleotides are labeled more efficiently with these systems than with other methods. In addition, end labeling of oligonucleotides is an economical alternative to having labels inserted during synthesis.
Both the 5′ EndTag and 3′ EndTag Nucleic Acid Labeling Systems enable the covalent attachment of a variety of fluorescent dyes, haptens, or affinity tags to the respective ends of a nucleic acid using thiol-specific chemistry. Labels containing thiol-reactive groups (maleimides, iodoacetamides, etc.) can easily be incorporated.
Labeling time is about 1 hour with very little hands-on time.
The 5′ EndTag System labels 5′ ends of DNA, RNA, or unmodified oligonucleotides. This kit is ideal for labeling PCR primers because a label is attached only to the 5′ end, leaving the 3′ end available for polymerization.
Labeling with the 5′ EndTag Kit is achieved in two steps:
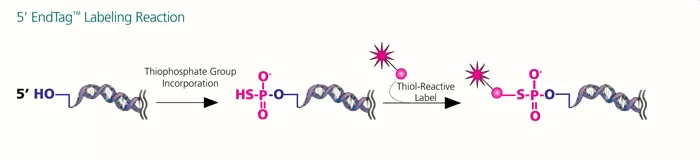
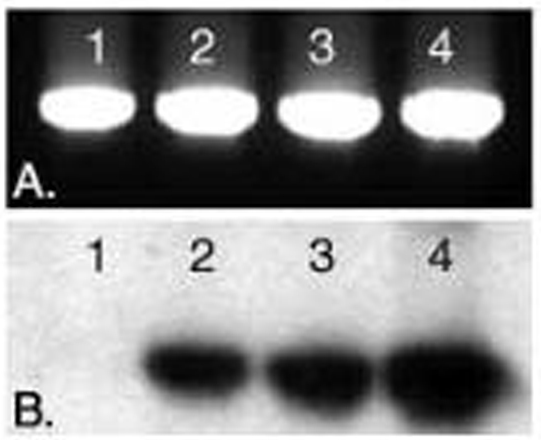
Figure 1: EtBr-stained gel of a 1002 bp PCR product amplified using:
Ln 1) Unlabeled primers
Ln 2) EndTag biotinylated forward primer and unlabeled reverse primer
Ln 3) Unlabeled forward primer and EndTag biotinylated reverse primer
Ln 4) EndTag biotinylated forward and reverse primers.
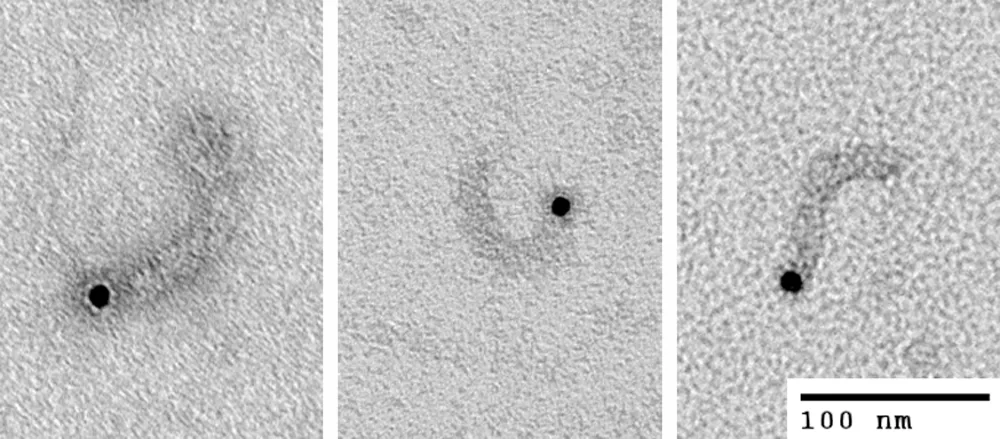
Figure 2: Transmission electron micrographs of molecules of influenza A viral ribonucleoprotein particles (vRNPs) labeled at the 5′ end of the vRNA with biotin using 5′ EndTag Kit, and further labeled with streptavidin gold. vRNPs from influenza A were purified according to Kemler et al., on a glycerol gradient with modifications as described in Wu et al. Courtesy of Drs. Winco WH Wu and Nelly Panté, University of British Columbia, Vancouver BC, Canada.
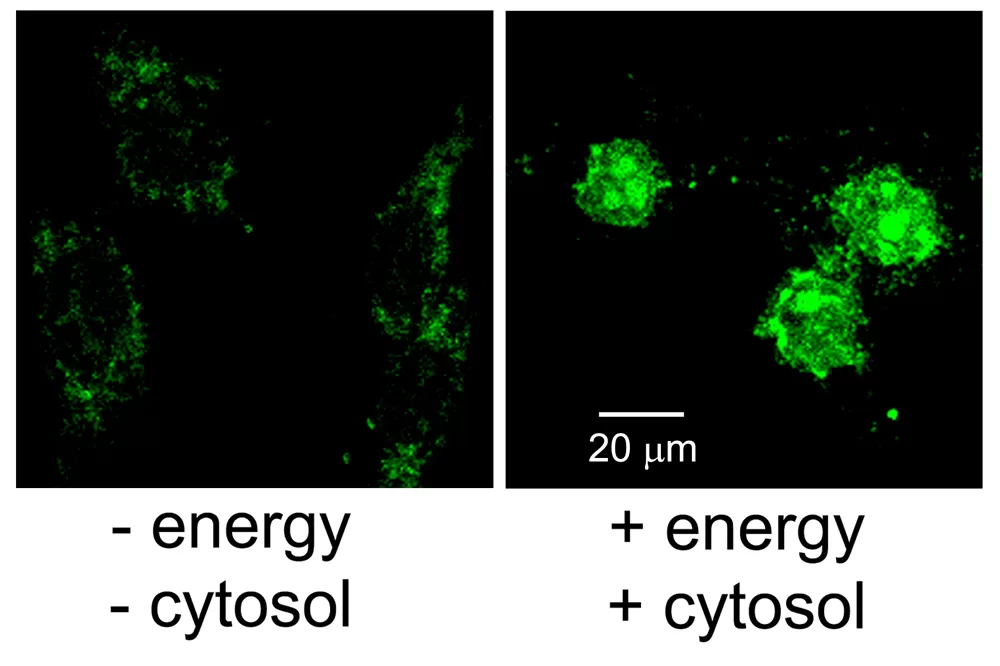
Figure 3: Nuclear import assay in digitonin-permeabilized HeLa cells of biotinylated vRNPs. vRNPs were labeled first at the 5′ end of the vRNA with biotin using the 5′ EndTag Kit and then with Vector Fluorescein Streptavidin. This allowed for direct fluorescence visualization of the vRNPs on a confocal fluorescence microscope. Nuclear import assays were carried out as described in Wu et al. The negative control consists of vRNPs added to the cells in the absence of energy and exogenous cytosol. In the presence of energy and cytosol, the fluorescein-labeled vRNPs successfully enter the nucleus, with a high degree of nucleolar staining. Courtesy of Drs. Winco WH Wu and Nelly Panté, University of British Columbia, Vancouver BC, Canada.
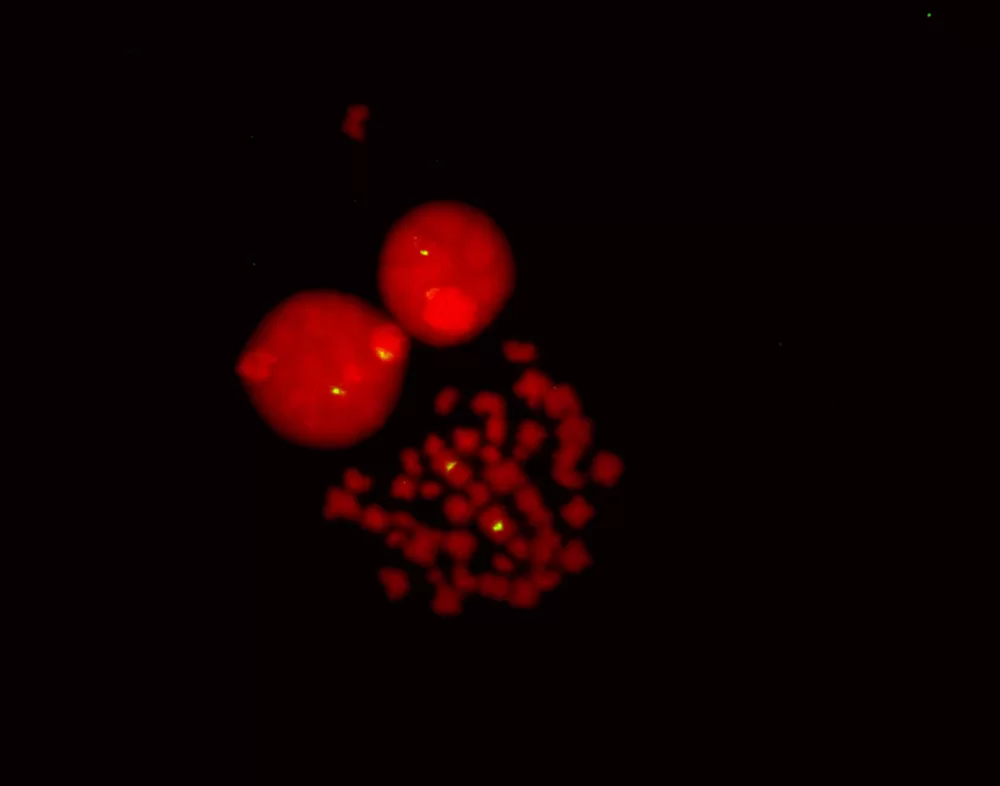
Figure 4: Fluorescence in situ hybridization of human chromosomes using 5′ EndTag Fluorescein-labeled pUC1.77 detected directly and mounted in Vectashield Mounting Media with PI.
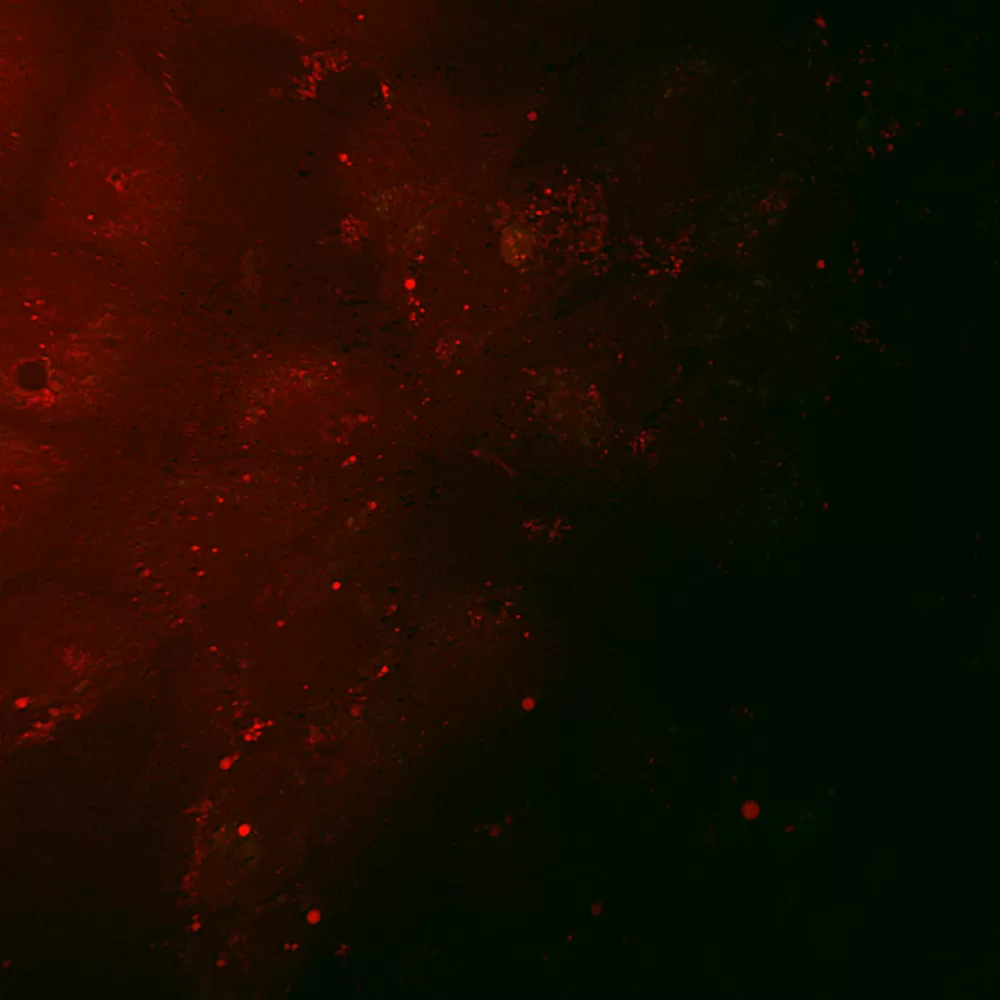
Figure 4: Coherent anti-Stokes Ramen scattering (CARS) and two-photon fluorescence (TPF) imaging of fluorescein-labeled HCV RNA inside Huh-7 cells. HCV RNA in-vitro transcripts were labeled with a fluorescein-maleimide label using the 5′ EndTag Nucleic Acid Labeling System. Huh-7 were transfected with 3µg of 5′-fluorescein-labeled HCV RNA and imaged at 4 h post-transfection. CARS and TPF of Mock transfected Huh-7 cells. Figure courtesy of Jennifer Haley, Sylvie Belanger, Adrian Pegoraro, Albert Stolow, and John P. Pezacki, The Steacie Institute for Molecular Sciences, The National Research Council of Canada. Unpublished data.
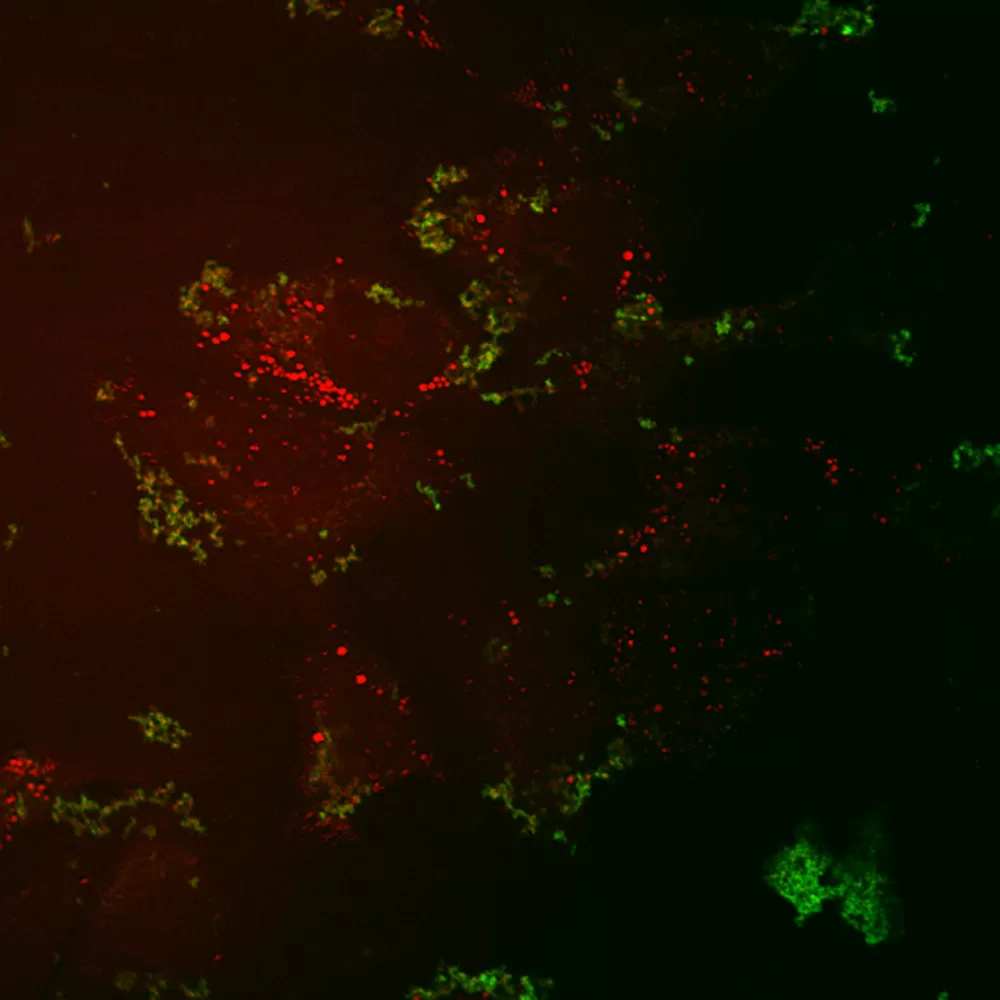
Figure 5: Coherent anti-Stokes Ramen scattering (CARS) and two-photon fluorescence (TPF) imaging of fluorescein-labeled HCV RNA inside Huh-7 cells. HCV RNA in-vitro transcripts were labeled with a fluorescein-maleimide label using the 5′ EndTag Nucleic Acid Labeling System. Huh-7 were transfected with 3µg of 5′-fluorescein-labeled HCV RNA and imaged at 4 h post-transfection. Here, CARS and TPF of Huh-7 cells transfected with fluorescein-labeled HCV RNA. Figure courtesy of Jennifer Haley, Sylvie Belanger, Adrian Pegoraro, Albert Stolow, and John P. Pezacki, The Steacie Institute for Molecular Sciences, The National Research Council of Canada. Unpublished data.
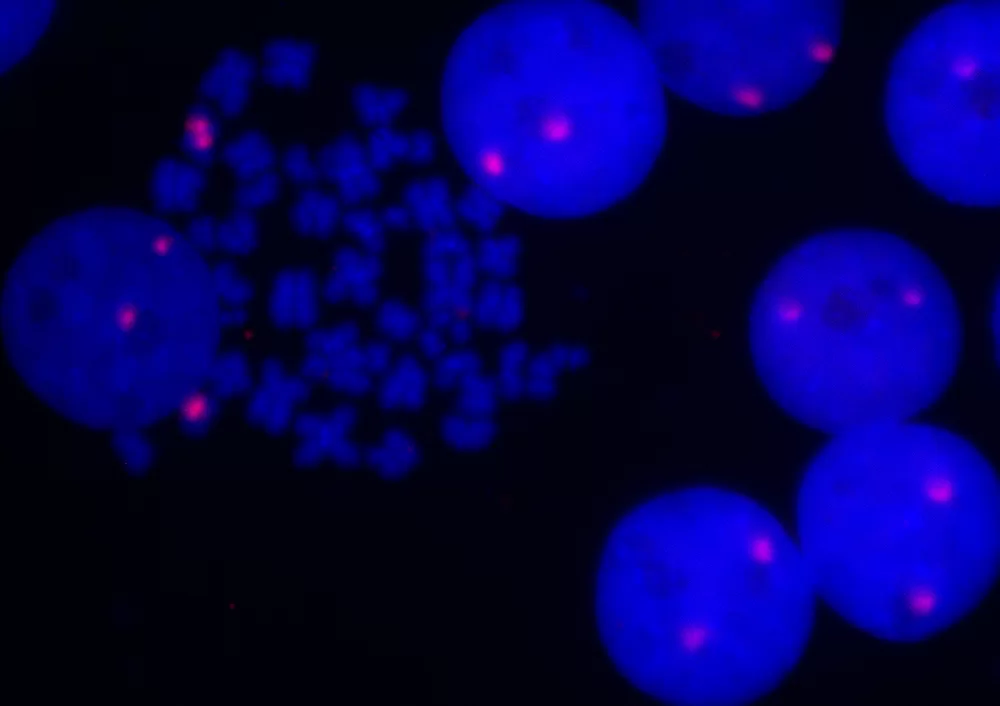
Figure 7: Fluorescence in situ hybridization of human chromosomes using 5′ EndTag Texas Red-labeled pHuR 98 detected directly and mounted in Vectashield Mounting Media with DAPI.
Applicable patents and legal notices are available at legal notices.



Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.
How do I Request a Quote?
To request a quote for products: