Click-&-Go® Lys-to-Lys Protein-Protein Conjugation Kit
Product No. 1008
The Lys-to-Lys Protein-Protein Conjugation Kit provides a sufficient amount of reagents to perform two protein-protein conjugation reactions. Any two lysine containing proteins (100-500 µg) in a volume of 100 µL (1-5 mg/mL) can be efficiently conjugated in less than 3 hours, start-to-finish.
Kit Contents
| Component | Amount |
|---|---|
| Tetrazine Reagent | 2 × 0.5 mg |
| TCO Reagent | 2 × 0.5 mg |
| DMSO | 1 mL |
| Saline Buffer Pack | 1 pack |
| Desalting Spin Columns | 8 × 0.5 mL |
Introduction
The Lys-to-Lys Protein-Protein Conjugation kit provides all of the necessary reagents to perform two protein-protein conjugation reactions. Conjugates are formed by the very efficient, catalyst-free bio-orthogonal ligation reaction between trans-cyclooctene (TCO) and tetrazine (Tz) functional groups. In the protocol provided, two proteins (or any other amine-containing biopolymers) are labeled separately with TCO and Tetrazine reagents. The labeled molecules are then mixed for 60 minutes in any suitable buffer to form a conjugate.
The extremely fast kinetics of the TCO/Tz ligation reaction enables rapid conjugation (30-60 min) of lysine containing proteins to each other at low concentrations (e.g. 5-10 µM), with > 99% conversion of the limiting protein in mild buffered media (e.g. PBS pH 7.5). Another benefit is the long-term stability of TCO and Tz functional groups on modified proteins stored in aqueous buffered media (e.g. maintaining > 90% reactivity after 1 month at 4°C, pH 7.5). This stability allows for worry-free conjugation results without the sensitive timing of reactions associated with classical thiol/maleimide chemistry.
Important information
- TCO and Tetrazine Reagents are moisture-sensitive. Avoid moisture condensation by allowing products to come to room temperature before opening.
- Prepare working stock solutions in aqueous buffers immediately before use. For protein labeling reactions, avoid buffers containing primary amines (e.g. Tris, glycine).
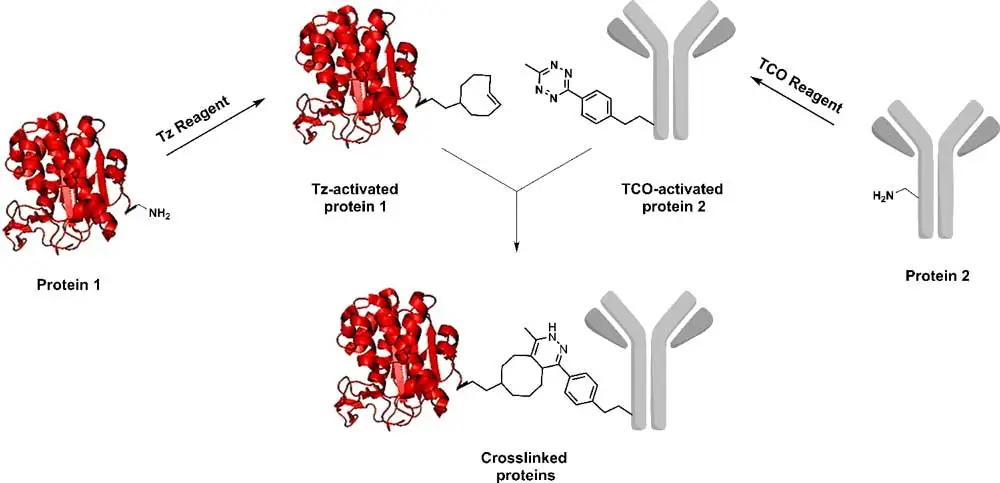
Figure 1. Schematic representation of protein-protein conjugation.
Protein requirements
- Protocol requires 100-500 µg of each protein in a volume of 0.1 mL (1-5 mg/mL)
- Proteins must be highly purified and their molecular weight known
- Proteins must have available primary amines (e.g. lysine residues)
Additional information
- When conjugating proteins at low concentrations (e.g. <5 µM), we recommend using a 1.5-2-fold molar excess over the limiting protein.
- Whenever possible, maintain the protein used in excess at a higher concentration than the limiting protein. For example, if IgG is the limiting protein at 1 mg/mL, keep the excess protein at 4-5 mg/mL for best results.
- Final protein-protein conjugates can be purified by a number of methods including size exclusion, ion exchange, affinity, or hydrophobic interaction chromatography. For certain applications, protein-protein conjugates can be used without further purification.
Materials Required but Not Provided
- UV-VIS spectrophotometer
- Microcentrifuge capable of handling 1.5 mL tubes
- Quartz semi-micro cuvette (50-100 µL)
- 1.5 mL microfuge tubes
- Pipettes and tips (P-10, P-100, P-1000)
- Ultrapure water (e.g. 18 MΩ-cm)
- Beaker, stir bar, and 6 N NaOH
- pH meter
Material preparation
A. Saline buffer preparation
- Dissolve saline buffer pack (provided) into 95 mL of ultrapure water. Adjust the pH of the solution to 7.5 with 6N NaOH. For long-term storage, sterile-filter the solution. Do not add sodium azide or Proclin 300 preservatives as these reagents interfere with protein determination (A280).
B. Protein preparation
- If protein #1 (100-500 µg) is lyophilized and free of exogenous amines (e.g. glycine or Tris), resuspend the protein in 100µL of the saline buffer (pH 7.5) to obtain a 1-5 mg/mL solution. Proceed to Tetrazine Labeling of Protein #1 as described in Section E.
- If protein #2 (100-500 µg) is lyophilized and free of exogenous amines (e.g. glycine or Tris), resuspend the protein in 100 µL of the saline buffer (pH 7.5) to obtain a 1-5 mg/mL solution. Proceed to TCO Labeling of Protein #2 as described in Section F.
- If either or both purified proteins (100-500 µg) are already in 100 µL of another buffer (e.g. PBS), buffer exchange into saline buffer (pH 7.5) will provide optimal results. This should be done prior to Tetrazine or TCO labeling, using the spin columns provided. See Step C and Step D for buffer exchange of proteins.
C. Spin column equilibration into saline buffer (pH 7.5) prior to buffer exchange
- Twist off the column’s bottom closure and loosen the cap. Place each 0.5 mL spin column into a clean 1.5 mL microfuge tube.
- Centrifuge column at 1,500 × g for 2 minutes to remove storage solution. Place a pen mark on the side of the column where the compacted resin is slanted upward. Place the column in centrifuge with the mark facing away from the center of the rotor in all subsequent centrifugation steps.
Note: resin will appear white in color and compacted after centrifugation. - Add 0.3 mL saline buffer (pH 7.5) to the top of each spin column, replace the cap and loosen.
- Centrifuge at 1,500 × g for 2 minutes to remove buffer.
- Repeat steps 3 and 4 two additional times, discarding buffer from the collection tube after each spin.
- Transfer the equilibrated spin column (resin should appear white and dry) into a new 1.5 ml microfuge tube and immediately proceed to the buffer exchange of the protein.
D. Buffer exchange of proteins
- Buffer exchange the protein into the saline buffer (pH 7.5) equilibrated spin column by slowly applying 100 µL of the protein solution to the center of equilibrated resin bed.
- Centrifuge at 1,500 × g for 2 minutes. Retain the eluate at the bottom of the 1.5 mL collection tube.
- Protein is now buffer exchanged.
Tetrazine labeling of protein #1 (limiting protein)
- Determine the volume of DMSO or water required to dissolve the tetrazine reagent provided (0.5 mg, MW = 686), and the amount of tetrazine stock solution needed for the labeling reaction using the Protein Labeling Calculator available on our product webpage. We recommend using the following excesses of tetrazine labeling reagent:
– For antibody concentrations from 0.5 mg/mL to 1 mg/mL, we recommend using a 10- to 20-fold excess of the tetrazine labeling reagent.
– For antibody concentrations from 1 mg/mL to 5 mg/mL, we recommend using a 5- to 10-fold excess of the tetrazine labeling reagent, depending on the expected degree of labeling. - Add the required volume of DMSO or water to the tetrazine reagent, vortex for 1 minute to completely dissolve.
- Add the calculated amount of tetrazine stock solution to 100 µL of buffer exchanged protein #1 (limiting protein).
- Allow reaction to proceed for 120 minutes to overnight at room temperature or 4°C.
- Remove any excess reagent from the labeled protein using a new buffer exchange spin column equilibrated in saline buffer or any other buffers by following Steps C and D. Retain the tetrazine-labeled protein #1 at the bottom of the collection tube.
- Determine the concentration of the tetrazine-modified protein #1 (mg/mL) by measuring A280.
Note: remove a 10 µL aliquot of tetrazine-modified protein and dilute into 90 µL of saline buffer, then measure A280 using a semi-micro quartz cuvette (50-100 µL). Alternatively, a Bradford or BCA protein assay can be performed using a 10 µL aliquot of the labeled protein. - The tetrazine-labeled protein is now ready for conjugation.
TCO labeling of protein #2 (excess protein)
- Determine the volume of DMSO required to dissolve the TCO reagent provided (0.5 mg, MW = 565), and the amount of TCO stock solution needed for the labeling reaction using the Protein Labeling Calculator available on our product webpage. We recommend using the following excesses of TCO labeling reagent:
– For antibody concentrations from 0.5 mg/mL to 1 mg/mL, we recommend using a 10- to 20-fold excess of TCO labeling reagent.
– For antibody concentrations from 1 mg/mL to 5 mg/mL, we recommend using a 5- to 10-fold excess of TCO labeling reagent, depending on the expected degree of labeling. - Add the required volume of DMSO to the TCO reagent, vortex for 2 minutes to completely dissolve.
- Add the calculated amount of TCO stock solution to 100 µL of buffer exchanged protein #2 (excess protein).
- Allow reaction to proceed for 120 minutes to overnight at room temperature or 4°C.
- Remove any excess reagent from the labeled protein using a new buffer exchange spin column equilibrated in saline buffer or any other buffers by following Steps C and D. Retain the TCO – labeled protein #2 at the bottom of the collection tube.
- Determine the concentration of the TCO – labeled protein #2 (mg/mL) by measuring A280.
Note: remove a 5 µL aliquot of TCO-modified protein and dilute into 95 µL saline buffer (e.g. 1:20), then measure A280 using a semi-micro quartz cuvette (50-100 µL). Alternatively, a Bradford or BCA protein assay can be used to determine the protein concentration if the protein’s E1% or molar extinction coefficient are unknown. - The TCO – labeled protein is now ready for conjugation.
Protein-protein conjugation
- Select the desired protein-protein stoichiometry for your conjugation reaction (e.g. 1:3).
Note: typical conditions use 1.2-1.5-fold molar excess of the excess protein over the limiting protein. - Using the selected stoichiometry, calculate the volume of TCO-modified protein (excess protein) to add to Tz-modified protein (limiting protein).
- Mix the required volume of TCO-modified protein with Tz-modified protein.
- Allow the conjugation reaction to proceed for 60 minutes at room temperature.
- Store protein-protein conjugates at 4°C until ready for use or purification.
Troubleshooting
| Problem | Possible Cause | Solution |
|---|---|---|
| No conjugation of tetrazine and TCO modified proteins | One or more proteins are not properly labeled with TCO or Tetrazine | Confirm purity and concentration of proteins prior to labeling process. Buffer exchange proteins into saline buffer (pH 7.5) if necessary. |
| TCO or Tetrazine Reagent is hydrolyzed | Allow product to equilibrate to room temperature before opening. | |
| Avoid buffers that contain primary amines such as Tris and glycine. Buffer exchange proteins before labeling if necessary. | ||
| Excess of labeling reagent improperly removed | Remove excess of unreacted tetrazine and TCO reagents by desalting. | |
| Low conjugation of TCO and Tetrazine labeled proteins | Suboptimal reaction conditions | Optimize conjugation conditions by altering molar excess |
| Confirm proper concentration of protein #1-Tz and protein #2-TCO prior to conjugation (i.e. 1-5 mg/mL) |

