Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
The R-PE Antibody Conjugation Kit is a flexible alternative to the R-PE Antibody All-in-One™ Conjugation Kit. Each kit contains sufficient material for two reactions of up to 1.3 mg antibody each. Based on SoluLINK® bioconjugation technology, the reactions are rapid and high-yielding, converting nearly 100% of antibody to PE conjugate. This kit is ideal for applications where purification to remove excess R-PE is either not required, or purification will be carried out by chromatographic methods (such as size exclusion FPLC).
| Conjugation Targets | Antibody |
|---|---|
| Label/Modifier Type | R-PE |
| Reactivity | Amine |
| Recommended Storage | 2° – 8° C — Do Not Freeze |
| Applications | Antibody Labeling |
The SoluLINK technology can be used to more easily and efficiently prepare R-PE-antibody conjugates as compared to alternative (maleimide/thiol-based) protocols. Other conjugation methods expose thiols on antibodies by DTT reduction of disulfide bonds, which cleaves the antibody into a variety of species. The SoluLINK bioconjugation technology, however, gently incorporates HyNic moieties on the intact antibody.
This technology is superior to the maleimide/thiol-based method in the following ways:
The SoluLINK bioconjugation technology is based on the formation of a stable bond formed by the reaction of an aromatic hydrazine and an aromatic aldehyde (Figure 1). S-HyNic is an amino-reactive modification reagent that directly converts amino groups on biomolecules and surfaces to HyNic groups. S-HyNic 1 (succinimidyl 6-hydrazinonicotinate acetone hydrazone, SANH) is used to incorporate aromatic hydrazine linkers on biomolecules. S- 4FB 2 (succinimidyl 4-formylbenzoate, SFB) is used to convert amino groups to aromatic aldehydes (4-formylbenzamide (4FB) groups). Addition of a HyNic-modified biomolecule to a 4FB-modified R-PE leads to the formation of the conjugate via a bis-arylhydrazone bond. The bis-aryl hydrazone bond is stable to 92°C and pH 2.0-10.0. The recommended pH for antibody conjugation is 6.0.
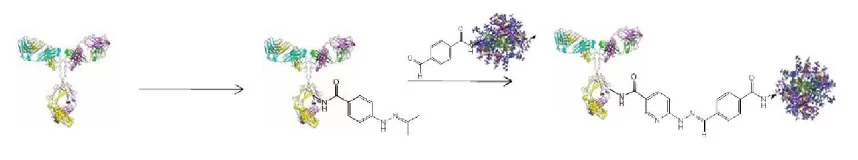
There are three crucial requirements that must be fulfilled for a reproducibly successful preparation of an R-PE Antibody conjugate using the SoluLINK bioconjugation technology:
*Dirksen, A., et al. Nucleophilic catalysis of hydrazone formation and transimination: implications for dynamic covalent chemistry. J Am Chem Soc, 2006. 128(49): p. 15602‐3.
SoluLINK® Bioconjugation For Research Use Only. Not for use in diagnostic procedures. For additional licensing restrictions, please see the license agreement at vectorlabs.com/solulink-research-license.
Products are for research use only, not for use in diagnostic or therapeutic procedures or for use in humans. Products are not for resale without express written permission of Seller. No license under any patent or other intellectual property right of Seller or its licensors is granted or implied by the purchase unless otherwise provided in writing.
Zeba and Thermo Scientific are trademarks of Thermo Fisher Scientific Inc.
Applicable patents and legal notices are available at legal notices.
Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.