
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Menu
Vector Laboratories is closed for the President’s Day on Monday, February 19th. We will be back in the office on Tuesday, February 20th.
We will respond to emails upon our return. Have a wonderful day.
Under perfect circumstances, chatting with a friend is clear and easy. But in a complex environment like a loud party, direct communication is often interrupted by competing sounds that drown out and muddle your speech. To get your message across, you need to find a way to cut through the background noise. Scientists performing antibody-based assays experience a similar challenge when analyzing their results at the end of a complicated staining protocol. To determine if what they are seeing is a real result, they need to block out the extraneous noise and focus on their specific signal.
Although immunohistochemistry (IHC) is a common technique, researchers often struggle with it due to the complexity of biological specimens. When performing IHC, scientists aspire to reduce background or non-specific staining on the specimen. The unwanted signal may be an overall weak stain across the tissue section or more intense coloration that appears specific and produces false positive results.
To gain a clear view, antibodies should bind their targets specifically, and the detection substrate should only precipitate at the site of antibody-bound antigens. However, non-specific binding of antibodies to tissue elements, such as charged particles, macromolecules, and Fc receptors, as well as endogenous enzyme activity often obscure specific antibody–antigen interactions. Blocking the sources of non-specific signals increases the signal-to-noise ratio for optimal results.
One major source of non-specific signal comes from intermolecular forces that promote antibody binding to other molecules in the specimen. To circumvent this, researchers add protein-based blocking agents that physically occupy non-specific binding sites prior to primary antibody addition. Depending on the blocking solution, the proteins within prevent non-specific binding to hydrophobic protein side chains or Fc receptors in the tissue. In theory, any protein that does not specifically bind to the epitope of interest can block unwanted interactions.
One common protein block is normal sera collected from healthy animals. Sera contain antibodies that bind non-specifically to Fc receptors, blocking the primary antibodies from interacting. The sera should originate from the species in which the secondary antibody was raised rather than that of the primary antibody animal. This ensures that the secondary antibody does not bind to the block and increase background signal.
Researchers also use protein block solutions, such as bovine serum albumin (BSA) and non-fat dry milk. BSA and non-fat dry milk proteins physically occupy binding spots that tend to interact non-specifically with antibodies through electrostatic and ionic interactions. An antibody specific for a target antigen should not have a greater affinity for the non-specific binding sites than the protein blockers. One advantage of this method is that there is no need to consider the source of the proteins in relation to the primary or secondary antibodies.
Animal-Free Blocker® is a universal blocking agent and diluent perfect for researchers trying to avoid animal-derived proteins. An alternative to sera, BSA, casein, and non-fat dry milk, this reagent can be used for many applications, including IHC, nucleic acid blotting, and protein blotting.
Chromogenic detection methods use an enzyme linked to a secondary antibody to bind primary antibodies bound to the target. The most popular IHC detection substrates interact with horseradish peroxidase (HRP)– and alkaline phosphatase (AP)–conjugated antibodies. However, these enzymes are already present within some tissues, where they react with the detection substrate and cause background signal. To quench enzyme activity, enzyme blocking agents must be added prior to the detection step. Researchers often perform this blocking step before primary antibody incubation.
Peroxidase is especially common in the kidney, liver, and tissues containing red blood cells. Researchers often quench endogenous peroxidase with hydrogen peroxide. By simply dropping the chemical diluted in water, phosphate-buffered saline, or methanol onto prepared slides, the resulting reaction inactivates the enzyme and converts hydrogen peroxide to oxygen and water. Aqueous hydrogen peroxide solutions may destroy the underlying tissue architecture if the tissue is peroxidase-rich. In this case, methanol would be the better solvent choice.
If the detection substrate reacts with alkaline phosphatase, researchers may block endogenous enzyme activity with levamisole solution. Rather than applying this block prior to primary antibody addition, levamisole solution should be added to the alkaline phosphatase substrate solution right before detection.
BLOXALL® Endogenous Blocking Solution is more effective than other enzyme blocking methods. It inactivates both endogenous peroxidase and alkaline phosphatase through a brief 10-minute incubation step. Many researchers prefer BLOXALL because it comes ready-to-use. Additionally, it is compatible with formalin-fixed, paraffin-embedded tissue sections, frozen tissue sections, and cell preparations.
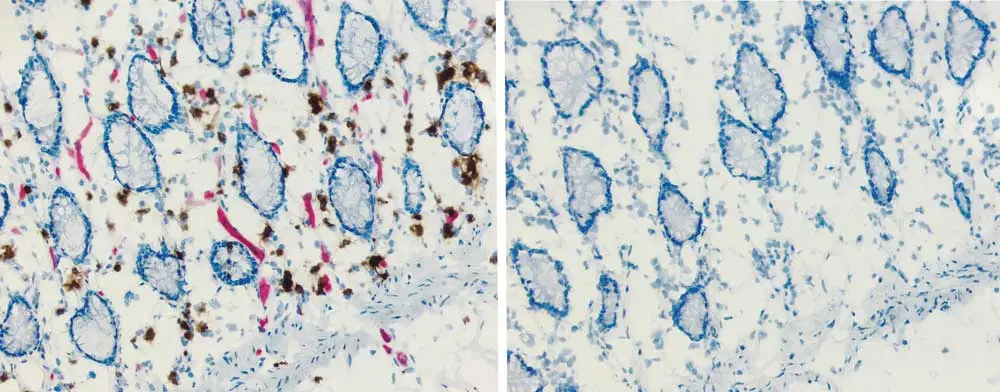
Biotin-based detection systems in combination with peroxidase or alkaline phosphatase improve the sensitivity of IHC. Avidin- or streptavidin-biotinylated enzyme complexes tightly bind biotinylated antibodies. However, biotin is present in many tissues and can generate a background signal. Blocking with unconjugated avidin/streptavidin and then biotin works to block endogenous biotin, biotin receptors, and avidin binding sites.
No single protein block strategy is effective for all IHC experiments, so running controls is always important. Researchers must consider their antibodies, tissue type, and detection reagents to choose the best blocks for their experiments. Sections from the same specimen may exhibit dissimilar behavior due to differences in tissue or cell type, which display different binding sites and enzymes.
To troubleshoot and identify the source of background, scientists should first omit the primary antibody and see if they have a non-specific signal. If so, they can adjust wash times, check for species cross-reactivity, or try different protein blockers and buffers. Through this series of troubleshooting steps, researchers can identify the conditions that produce the best IHC results.
For more helpful tips and tricks be sure to check out our IHC Resource Guide.





Stay in the Loop. Join Our Online Community
Together we breakthroughTM

©Vector Laboratories, Inc. 2024 All Rights Reserved.